Amino acids
4.63/5 (8)
Amino acids are biologically important organic compounds made from amine and carboxylic acid functional groups, along with a side-chain specific to each amino acid.
The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen, although other elements such as sulfur are found in the side-chains of certain amino acids.
In the form of proteins, amino acids comprise the second-largest component – apart from water – in human muscles, cells and other tissues.
Because of their biological significance, amino acids are essential in nutrition and are commonly used in nutritional supplements.
Standard Amino Acids
Twenty amino acids are known as “standard” amino acids, which are encoded directly from the genetic code.
Outside proteins, amino acids also perform critical biological roles including neurotransmitting, transport, and synthesis of special compounds.
For example the standard glutamic acid and the non-standard gamma-amino acid: gamma-amino-butyric acid (GABA) are the brain’s main excitatory and inhibitory neurotransmitters, respectively.
Hydroxyproline – a major component of the connective tissue collagen – is synthesised from proline.
The standard amino acid glycine is used to synthesise porphyrins used in red blood cells, and the non-standard carnitine is used in lipid transport.
Essential Amino Acids
Nine of the 20 standard amino acids are called “essential” amino acids for humans because they cannot be created from other compounds by the human body. These amino acids, which must be present in the food, are: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine.
Conditionally Essential Amino Acids
Six other amino acids are considered conditionally essential in the human diet, meaning their synthesis can be limited under special pathophysiological conditions, such as prematurity in the infant or individuals in severe catabolic distress. These six are arginine, cysteine, glycine, glutamine, proline, and tyrosine.
Non-Essential Amino Acids
Five amino acids are not essential in humans since they can be synthesized in sufficient quantities in the body. These five are alanine, asparagine, aspartic acid, glutamic acid and serine.
Amino Acid Structure
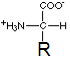 In the structure shown here, R represents a side-chain specific to each amino acid. Depending on their side-chain the amino acids have different characteristics. The side-chain can make an amino acid a weak acid or a weak base, and hydrophilic if the side-chain is polar or hydrophobic if it is nonpolar.
In the structure shown here, R represents a side-chain specific to each amino acid. Depending on their side-chain the amino acids have different characteristics. The side-chain can make an amino acid a weak acid or a weak base, and hydrophilic if the side-chain is polar or hydrophobic if it is nonpolar.
The hydrophobic amino acids tend to repel the aqueous environment and, therefore, reside predominantly in the interior of proteins. These amino acids neither ionize nor participate in the formation of H-bonds.
The hydrophilic amino acids tend to interact with the aqueous environment, they are often involved in the formation of H-bonds and are predominantly found on the exterior surfaces proteins or in the reactive centers of enzymes.
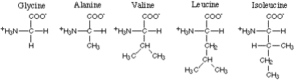 The amino acids with nonpolar hydrophobe aliphatic R-groups comprise glycine, alanine, valine, aliphatic amino acids leucine and isoleucine.
The amino acids with nonpolar hydrophobe aliphatic R-groups comprise glycine, alanine, valine, aliphatic amino acids leucine and isoleucine.
Of these valine, leucine and isoleucine have a branched-chain R-group – they are therefore termed “branched-chain amino acids” or BCAA.
–
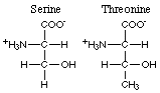 The non-aromatic amino acids with polar hydroxyl R-groups comprise serine and threonine.
The non-aromatic amino acids with polar hydroxyl R-groups comprise serine and threonine.
–
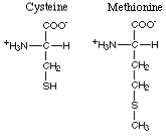 The amino acids with nonpolar sulfur-containing R-groups comprise cysteine and methionine.
The amino acids with nonpolar sulfur-containing R-groups comprise cysteine and methionine.
–
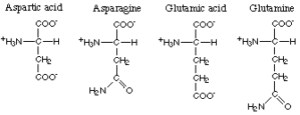 The amino acids with polar acid or corresponding polar amide R-groups comprise aspartic acid, asparagine, glutamic acid and glutamine.
The amino acids with polar acid or corresponding polar amide R-groups comprise aspartic acid, asparagine, glutamic acid and glutamine.
–
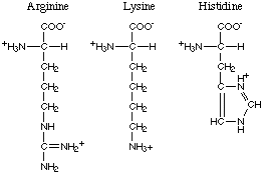 The amino acids with polar basic R-groups comprise arginine, lysine and histidine.
The amino acids with polar basic R-groups comprise arginine, lysine and histidine.
–
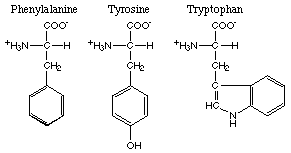 The amino acids with aromatic R-groups comprise phenylalanine, tyrosine, and tryptophan. Of these phenylalanine and tryptophan are nonpolar and tyrosine is polar.
The amino acids with aromatic R-groups comprise phenylalanine, tyrosine, and tryptophan. Of these phenylalanine and tryptophan are nonpolar and tyrosine is polar.
–
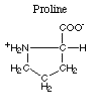 The amino acid with a nonpolar imino type R-group is proline.
The amino acid with a nonpolar imino type R-group is proline.
–
–
Amino Acid Function
It is the nature of the amino acid R-groups that dictate structure-function relationships of peptides and proteins.
The hydrophobic amino acids will generally be encountered in the interior of proteins shielded from direct contact with water.
Conversely, the hydrophilic amino acids are generally found on the exterior of proteins as well as in the active centers of enzymatically active proteins. Indeed, it is the very nature of certain amino acid R-groups that allow enzyme reactions to occur.
The imidazole ring of histidine allows it to act as either a proton donor or acceptor at physiological pH. Hence, it is frequently found in the reactive center of enzymes.
Equally important is the ability of histidines in hemoglobin to buffer the H+ ions from carbonic acid ionization in red blood cells. It is this property of hemoglobin that allows it to exchange oxygen (O2) and carbon dioxide (CO2) at the tissues or lungs, respectively.
The primary alcohol of serine and threonine as well as the thiol (–SH) of cysteine allow these amino acids to act in enzymatic catalysis.
Additionally, the thiol of cysteine is able to form a disulfide bond with other cysteines. This simple disulfide is identified as cystine.
The formation of disulfide bonds between cysteines present within proteins is important to the formation of active structural domains in a large number of proteins.
Disulfide bonding between cysteines in different polypeptide chains plays a crucial role in ordering the structure of complex proteins, e.g. the insulin receptor.
Alanine
Alanine (symbol Ala) is an α-amino acid that is used in the biosynthesis of proteins. It is non-essential in humans. Since the body can synthesize it, it does not need to be present in the diet.
The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-Alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins.
Structure
Alanine is an aliphatic amino acid, because the side-chain connected to the α-carbon atom is a methyl group (-CH3), making it the simplest α-amino acid except for glycine. The methyl side-chain of alanine is non-reactive and is therefore hardly ever directly involved in protein function.
Dietary sources
Alanine is found in a wide variety of foods, but is particularly concentrated in meats. See also table below.
Biosynthesis
Alanine can be synthesized from pyruvate and branched chain amino acids such as valine, leucine, and isoleucine.
Alanine is most commonly produced by reductive amination of pyruvate, a two-step process.
Because transamination reactions are readily reversible and pyruvate is present in all cells, alanine can be easily formed and thus has close links to metabolic pathways such as glycolysis, gluconeogenesis, and the citric acid cycle.
Physiological function
Glucose–alanine cycle
In mammals, alanine plays a key role in glucose–alanine cycle between tissues and liver.
In muscle and other tissues that degrade amino acids for fuel, amino groups are collected in the form of glutamate by transamination.
Glutamate can then transfer its amino group to pyruvate, a product of muscle glycolysis, through the action of alanine aminotransferase, forming alanine and α-ketoglutarate.
The alanine enters the bloodstream, and is transported to the liver. The alanine aminotransferase reaction takes place in reverse in the liver, where the regenerated pyruvate is used in gluconeogenesis, forming glucose which returns to the muscles through the circulation system.
Glutamate in the liver enters mitochondria and is broken down by glutamate dehydrogenase into α-ketoglutarate and ammonium, which in turn participates in the urea cycle to form urea which is excreted through the kidneys.
The glucose–alanine cycle enables pyruvate and glutamate to be removed from the muscle and safely transported to the liver, where glucose is regenerated from pyruvate and then returned to muscle.
This moves the energetic burden of gluconeogenesis to the liver instead of the muscle, and all available ATP in the muscle can be devoted to muscle contraction. It is a catabolic pathway, and relies upon protein breakdown in the muscle tissue.
Link to diabetes
Alterations in the alanine cycle that increase the levels of serum alanine aminotransferase (ALT) are linked to the development of type II diabetes.
–
Below is a list of foods having the highest content of alanine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
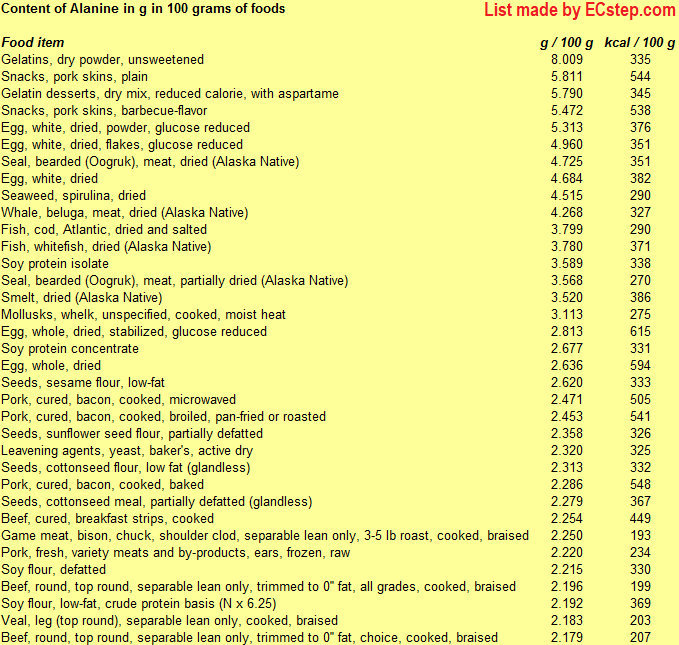
Reference 1
Arginine
Arginine also knows as L-arginine (symbol Arg) is an α-amino acid that is used in the biosynthesis of proteins. At physiological pH arginine is a charged, aliphatic amino acid. It is the precursor for the biosynthesis of nitric oxide.
In humans, arginine is classified as a semiessential or conditionally essential amino acid, depending on the developmental stage and health status of the individual. Preterm infants are unable to synthesize or create arginine internally, making the amino acid nutritionally essential for them.
Most healthy people do not need to supplement with arginine because it is a component of all protein-containing foods and can be synthesized in the body from glutamine via citrulline.
Dietary sources
Arginine is a conditionally essential amino acid in humans, as it may be required depending on the health status or lifecycle of the individual.
For example, while healthy adults can supply their own requirement for arginine, immature and rapidly growing individuals require arginine in their diet, and it is also essential under physiological stress, for example during recovery from burns, injury, and sepsis, or when the small intestine and kidneys, which are the major sites of arginine biosynthesis, have been damaged.
Sources of arginine include meat, dairy products, and eggs, seeds of all types, for example grains, beans, and nuts. See also table below.
Biosynthesis
Arginine is synthesized from citrulline in arginine and proline metabolism by the sequential action of the cytosolic enzymes argininosuccinate synthetase and argininosuccinate lyase. This is an energetically costly process, because for each molecule of argininosuccinate that is synthesized, one molecule of adenosine triphosphate (ATP) is hydrolyzed to adenosine monophosphate (AMP), consuming two ATP equivalents.
Citrulline can be derived from multiple sources:
- from arginine itself via nitric oxide synthase, as a byproduct of the production of nitric oxide for signaling purposes
- from ornithine through the breakdown of proline or glutamine/glutamate
- from asymmetric dimethylarginine via DDAH
The pathways linking arginine, glutamine, and proline are bidirectional. Thus, the net use or production of these amino acids is highly dependent on cell type and developmental stage.
On a whole-body basis, synthesis of arginine occurs principally via the intestinal–renal axis: the epithelial cells of the small intestine produce citrulline, primarily from glutamine and glutamate, which is carried in the bloodstream to the proximal tubule cells of the kidney, which extract citrulline from the circulation and convert it to arginine, which is returned to the circulation.
This means that impaired small bowel or renal function can reduce arginine synthesis, increasing the dietary requirement.
Synthesis of arginine from citrulline also occurs at a low level in many other cells, and cellular capacity for arginine synthesis can be markedly increased under circumstances that increase the production of inducible NOS.
This allows citrulline, a byproduct of the NOS-catalyzed production of nitric oxide, to be recycled to arginine in a pathway known as the citrulline-NO or arginine-citrulline pathway.
This is demonstrated by the fact that, in many cell types, NO synthesis can be supported to some extent by citrulline, and not just by arginine.
This recycling is not quantitative, however, because citrulline accumulates in NO-producing cells along with nitrate and nitrite, the stable end-products of NO breakdown.
Function
Arginine plays an important role in cell division, wound healing, removing ammonia from the body, immune function, and the release of hormones. It is a precursor for the synthesis of nitric oxide (NO), making it important in the regulation of blood pressure.
Proteins
Arginine’s side chain is amphipathic, because at physiological pH it contains a positively charged guanidinium group, which is highly polar, at the end of a hydrophobic aliphatic hydrocarbon chain.
Because globular proteins have hydrophobic interiors and hydrophilic surfaces, arginine is typically found on the outside of the protein, where the hydrophilic head group can interact with the polar environment, for example taking part in hydrogen bonding and salt bridges.
For this reason, it is frequently found at the interface between two proteins. The aliphatic part of the side chain sometimes remains below the surface of the protein.
Arginine residues in proteins can be deiminated by PAD enzymes to form citrulline, in a post-translational modification process called citrullination.
This is important in fetal development, is part of the normal immune process, as well as the control of gene expression, but is also significant in autoimmune diseases. Another post-translational modification of arginine involves methylation by protein methyltransferases.
Precursor
Arginine is the immediate precursor of NO, an important signaling molecule which can act as a second messenger, as well as an intercellular messenger which regulates vasodilation, and also has functions in the immune system’s reaction to infection.
Arginine is also a precursor for urea, ornithine, and agmatine; is necessary for the synthesis of creatine; and can also be used for the synthesis of polyamines mainly through ornithine and to a lesser degree through agmatine, citrulline, and glutamate.
The presence of asymmetric dimethylarginine (ADMA), a close relative, inhibits the nitric oxide reaction; therefore, ADMA is considered a marker for vascular disease, just as L-arginine is considered a sign of a healthy endothelium.
Safety
L-arginine is generally recognized as safe at intakes of up to 20 grams per day.
Structure
The amino acid side-chain of arginine consists of a 3-carbon aliphatic straight chain, the distal end of which is capped by a guanidinium group, which has a pKa of 12.48, and is therefore always protonated and positively charged at physiological pH.
Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized, enabling the formation of multiple hydrogen bonds.
Research
Growth hormone
Intravenously-administered arginine is used in growth hormone stimulation tests because it stimulates the secretion of growth hormone. A review of clinical trials concluded that oral arginine increases growth hormone, but decreases growth hormone secretion, which is normally associated with exercising. However, a more recent trial reported that although oral arginine increased plasma levels of L-arginine it did not cause an increase in growth hormone.
High blood pressure
A meta-analysis showed that L-arginine reduces blood pressure with pooled estimates of 5.4 mmHg for systolic blood pressure and 2.7 mmHg for diastolic blood pressure.
Supplementation with L-arginine reduces diastolic blood pressure and lengthens pregnancy for women with gestational hypertension, including women with high blood pressure as part of pre-eclampsia. It did not lower systolic blood pressure or improve weight at birth.
–
Below is a list of foods having the highest content of arginine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
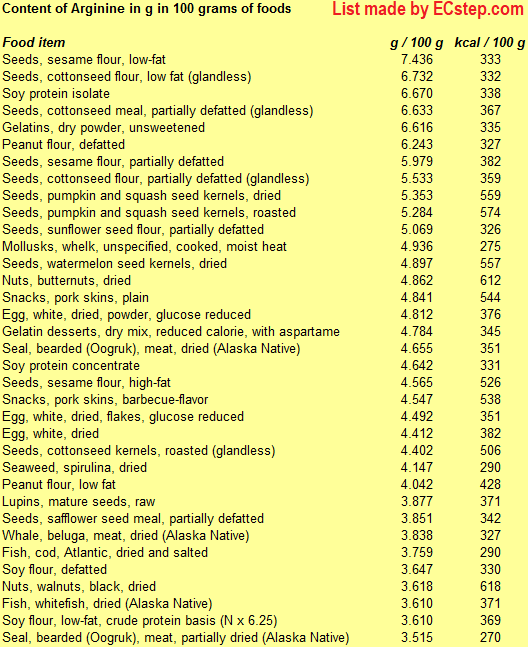
Reference 1
Asparagine
Asparagine (symbol Asn), is an α-amino acid that is used in the biosynthesis of proteins. It is non-essential in humans, meaning the body can synthesize it.
A reaction between asparagine and reducing sugars or other source of carbonyls produces acrylamide (a potential carcinogen) in food when heated to sufficient temperature. These products occur in baked goods such as French fries, potato chips, and toasted bread.
Asparagine was first isolated from asparagus juice, in which it is abundant, hence the chosen name. Liquorice root does also contain asparagine.
Structural function in proteins
Since the asparagine side-chain can form hydrogen bond interactions with the peptide backbone, asparagine residues are often found near the beginning of alpha-helices or in beta sheets.
Dietary sources
Asparagine is found in dairy, whey, beef, poultry, eggs, fish, lactalbumin, seafood, asparagus, potatoes, legumes, nuts, seeds, soy, whole grains. See also table below.
Biosynthesis
The precursor to asparagine is oxaloacetate. Oxaloacetate is converted to aspartate using a transaminase enzyme.
Degradation
Asparagine usually enters the citric acid cycle in humans as oxaloacetate.
Function
Asparagine is required for development and function of the brain. It also plays an important role in the synthesis of ammonia.
According to a 2018 article in The Guardian, a study found that decreasing levels of asparagine “dramatically” reduced the spread of breast cancer in laboratory mice. The article noted that similar studies had not been conducted in humans.
–
Below is a list of foods having the highest content of asparagine and aspartic acid (taken together since the acid forms are easily converted to the amide forms and vice versa) in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
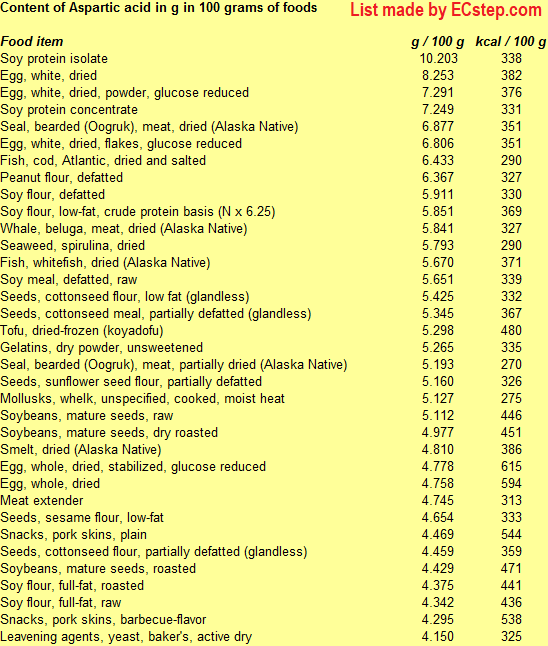
Reference 1
Aspartic acid
Aspartic acid (symbol Asp) is an α-amino acid that is used in the biosynthesis of proteins. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed.
Biosynthesis
Because Aspartate can be synthesized by the body it is classified as a non-essential amino acid. In the human body, aspartate is most frequently synthesized through the transamination of oxaloacetate. The biosynthesis of aspartate is facilitated by an aminotransferase enzyme: the transfer of an amine group from another molecule such as alanine or glutamine yields aspartate and an alpha-keto acid.
Aspartate also plays an important role in the urea cycle.
Participation in the urea cycle
In the urea cycle, aspartate and ammonia donate amino groups leading to the formation of urea.
Other biochemical roles
Aspartate has many other biochemical roles. It is a metabolite in the urea cycle and participates in gluconeogenesis. Aspartate donates one nitrogen atom in the biosynthesis of inosine, the precursor to the purine bases. In addition, aspartic acid acts as a hydrogen acceptor in a chain of ATP synthase.
Neurotransmitter
Aspartate (the conjugate base of aspartic acid) stimulates NMDA receptors, though not as strongly as the amino acid neurotransmitter L-glutamate does.
Current applications include biodegradable polymers (polyaspartic acid), low calorie sweeteners (aspartame), scale and corrosion inhibitors, and resins.
–
Below is a list of foods having the highest content of asparagine and aspartic acid (taken together since the acid forms are easily converted to the amide forms and vice versa) in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.

Reference 1
Cysteine
Cysteine (symbol Cys) is a conditionally essential proteinogenic amino acid. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920.
Cysteine has the same structure as serine, but with one of its oxygen atoms replaced by sulfur; replacing it with selenium gives selenocysteine.
Dietary sources
Although classified as a semi-essential amino acid, in rare cases, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes.
Cysteine can usually be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available.
Cysteine is catabolized in the gastrointestinal tract and blood plasma. In contrast, cystine travels safely through the GI tract and blood plasma and is promptly reduced to the two cysteine molecules upon cell entry.
Cysteine is found in most high-protein foods, including meat (including pork and poultry), eggs, dairy. It is also found in red peppers, garlic, onions, broccoli, brussels sprout, oats, wheat germ, and sprouted lentils. See also table below.
Biosynthesis
In animals, biosynthesis begins with the amino acid serine. The sulfur is derived from methionine, which is converted to homocysteine through the intermediate S-adenosylmethionine. Cystathionine beta-synthase then combines homocysteine and serine to form the asymmetrical thioether cystathionine.
The enzyme cystathionine gamma-lyase converts the cystathionine into cysteine and alpha-ketobutyrate.
Biological functions
The cysteine sulfhydryl group is nucleophilic and easily oxidized. The reactivity is enhanced when the thiol is ionized, and cysteine residues in proteins have pKa values close to neutrality, so are often in their reactive thiolate form in the cell. Because of its high reactivity, the sulfhydryl group of cysteine has numerous biological functions, and cysteine may have played an important role in the development of primitive life on Earth.
Precursor to the antioxidant glutathione
Due to the ability of thiols to undergo redox reactions, cysteine has antioxidant properties. Cysteine’s antioxidant properties are typically expressed in the tripeptide glutathione, which occurs in humans as well as other organisms. The systemic availability of oral glutathione (GSH) is negligible; so it must be biosynthesized from its constituent amino acids, cysteine, glycine and glutamic acid.
Glutamic acid and glycine are readily available in most Western diets, but the availability of cysteine can be the limiting substrate.
Precursor to iron-sulfur clusters
Cysteine is an important source of sulfide in human metabolism. The sulfide in iron-sulfur clusters and in nitrogenase is extracted from cysteine, which is converted to alanine in the process.
Metal ion binding
Beyond the iron-sulfur proteins, many other metal cofactors in enzymes are bound to the thiolate substituent of cysteinyl residues. Examples include zinc in zinc fingers and alcohol dehydrogenase, copper in the blue copper proteins, iron in cytochrome P450 and nickel in the [NiFe]-hydrogenases. The sulfhydryl group also has a high affinity for heavy metals, so that proteins containing cysteine, such as metallothionein, will bind metals such as mercury, lead and cadmium tightly.
Roles in protein structure
Cysteine has traditionally been considered to be a hydrophilic amino acid, based largely on the chemical parallel between its sulfhydryl group and the hydroxyl groups in the side-chains of other polar amino acids.
However, the cysteine side chain has been shown to stabilize hydrophobic interactions in micelles to a greater degree than the side chain in the non-polar amino acid glycine and the polar amino acid serine.
While free cysteine residues do occur in proteins, most are covalently bonded to other cysteine residues to form disulfide bonds. Disulfide bonds play an important role in the folding and stability of some proteins, usually proteins secreted to the extracellular medium. Since most cellular compartments are reducing environments, disulfide bonds are generally unstable in the cytosol with some exceptions as noted below.
Disulfide bonds in proteins are formed by oxidation of the sulfhydryl group of cysteine residues. The other sulfur-containing amino acid, methionine, cannot form disulfide bonds. Cysteine residues play a valuable role by crosslinking proteins, which increases the rigidity of proteins and also functions to confer proteolytic resistance (since protein export is a costly process, minimizing its necessity is advantageous).
Insulin is an example of a protein with cystine crosslinking, wherein two separate peptide chains are connected by a pair of disulfide bonds.
Cysteine is a precursor in the food, pharmaceutical and personal-care industries. One of the largest applications is the production of flavors. For example, the reaction of cysteine with sugars in a Maillard reaction yields meat flavors. L-Cysteine is also used as a processing aid for baking.
In the field of personal care, cysteine is used for permanent wave applications, predominantly in Asia. Again, the cysteine is used for breaking up the disulfide bonds in the hair’s keratin.
Reducing toxic effects of alcohol
Cysteine has been proposed as a preventative or antidote for some of the negative effects of alcohol, including liver damage and hangover. It counteracts the poisonous effects of acetaldehyde. Cysteine supports the next step in metabolism, which turns acetaldehyde into the relatively harmless acetic acid. No direct evidence indicates its effectiveness in humans who consume alcohol at low levels.
N-Acetylcysteine
N-Acetyl-L-cysteine is a derivative of cysteine wherein an acetyl group is attached to the nitrogen atom. This compound is sold as a dietary supplement, and used as an antidote in cases of acetaminophen overdose.
Sheep
Cysteine is required by sheep to produce wool: It is an essential amino acid that must be taken in from their feed. As a consequence, during drought conditions, sheep produce less wool; however, transgenic sheep that can make their own cysteine have been developed.
Dietary restrictions
The animal-originating sources of L-cysteine as a food additive are a point of contention for people following dietary restrictions such as Kosher, Halal, Vegan or Vegetarian. To avoid this problem, L-cysteine can also be sourced from microbial or other synthetic processes.
–
Below is a list of foods having the highest content of nonpolar sulfur-
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
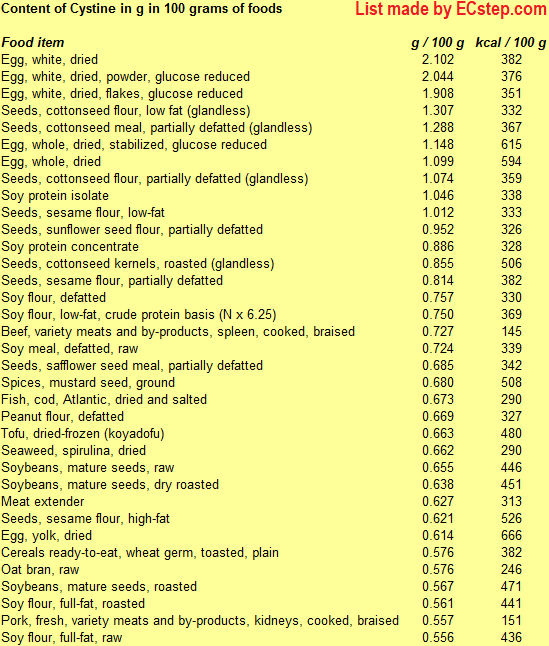
Reference 1
Glutamic acid
Glutamic acid (symbol Glu) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is non-essential in humans, meaning the body can synthesize it. It is also an excitatory neurotransmitter, in fact the most abundant one, in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons.
Although they occur naturally in many foods, the flavor contributions made by glutamic acid and other amino acids were only scientifically identified early in the twentieth century.
In 1908 Japanese researcher Kikunae Ikeda of the Tokyo Imperial University identified brown crystals left behind after the evaporation of a large amount of kombu broth as glutamic acid. These crystals, when tasted, reproduced the ineffable but undeniable flavor he detected in many foods, most especially in seaweed. Professor Ikeda termed this flavor umami. He then patented a method of mass-producing a crystalline salt of glutamic acid, monosodium glutamate.
Metabolism
Glutamate is a key compound in cellular metabolism. In humans, dietary proteins are broken down by digestion into amino acids, which serve as metabolic fuel for other functional roles in the body. A key process in amino acid degradation is transamination, in which the amino group of an amino acid is transferred to an α-ketoacid, typically catalysed by a transaminase.
A very common α-keto acid is α-ketoglutarate, an intermediate in the citric acid cycle. Transamination of α-ketoglutarate gives glutamate. The resulting α-ketoacid product is often a useful one as well, which can contribute as fuel or as a substrate for further metabolism processes.
Both pyruvate and oxaloacetate are key components of cellular metabolism, contributing as substrates or intermediates in fundamental processes such as glycolysis, gluconeogenesis, and the citric acid cycle.
Glutamate also plays an important role in the body’s disposal of excess or waste nitrogen. Glutamate undergoes deamination, an oxidative reaction catalysed by glutamate dehydrogenase, as follows:
glutamate + H2O + NADP+ → α-ketoglutarate + NADPH + NH3 + H+
Ammonia (as ammonium) is then excreted predominantly as urea, synthesised in the liver. Transamination can thus be linked to deamination, effectively allowing nitrogen from the amine groups of amino acids to be removed, via glutamate as an intermediate, and finally excreted from the body in the form of urea.
Glutamate is also a neurotransmitter, which makes it one of the most abundant molecules in the brain. Malignant brain tumors known as glioma or glioblastoma exploit this phenomenon by using glutamate as an energy source, especially when these tumors become more dependent on glutamate due to mutations.
Neurotransmitter
Glutamate is the most abundant excitatory neurotransmitter in the vertebrate nervous system. At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger release of glutamate from the presynaptic cell. In the opposing postsynaptic cell, glutamate receptors, such as the NMDA receptor or the AMPA receptor, bind glutamate and are activated.
Because of its role in synaptic plasticity, glutamate is involved in cognitive functions such as learning and memory in the brain.
The form of plasticity known as long-term potentiation takes place at glutamatergic synapses in the hippocampus, neocortex, and other parts of the brain.
Glutamate works not only as a point-to-point transmitter, but also through spill-over synaptic crosstalk between synapses in which summation of glutamate released from a neighboring synapse creates extrasynaptic signaling/volume transmission.
In addition, glutamate plays important roles in the regulation of growth cones and synaptogenesis during brain development.
Brain nonsynaptic glutamatergic signaling circuits
A gene expressed in glial cells actively transports glutamate into the extracellular space, while, in the nucleus accumbens-stimulating group II metabotropic glutamate receptors, this gene was found to reduce extracellular glutamate levels. This raises the possibility that this extracellular glutamate plays an “endocrine-like” role as part of a larger homeostatic system.
GABA precursor
Glutamate also serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. This reaction is catalyzed by glutamate decarboxylase (GAD), which is most abundant in the cerebellum and pancreas.
Stiff person syndrome is a neurologic disorder caused by anti-GAD antibodies, leading to a decrease in GABA synthesis and, therefore, impaired motor function such as muscle stiffness and spasm.
Since the pancreas has abundant GAD, a direct immunological destruction occurs in the pancreas and the patients will have diabetes mellitus.
Flavor enhancer
Glutamic acid, being a constituent of protein, is present in foods that contain protein, but it can only be tasted when it is present in an unbound form. Significant amounts of free glutamic acid are present in a wide variety of foods, including cheese and soy sauce, and is responsible for umami, one of the five basic tastes of the human sense of taste. Glutamic acid is often used as a food additive and flavor enhancer in the form of its sodium salt, known as monosodium glutamate (MSG).
Nutrient
All meats, poultry, fish, eggs, dairy products, and kombu are excellent sources of glutamic acid. Some protein-rich plant foods also serve as sources. 30% to 35% of gluten (much of the protein in wheat) is glutamic acid. Ninety-five percent of the dietary glutamate is metabolized by intestinal cells in a first pass. See also table below.
–
Below is a list of foods having the highest content of glutamic acid and glutamine taken together (since the acid forms are easily converted to the amide forms and vice versa) in g per 100 grams of food.
. Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
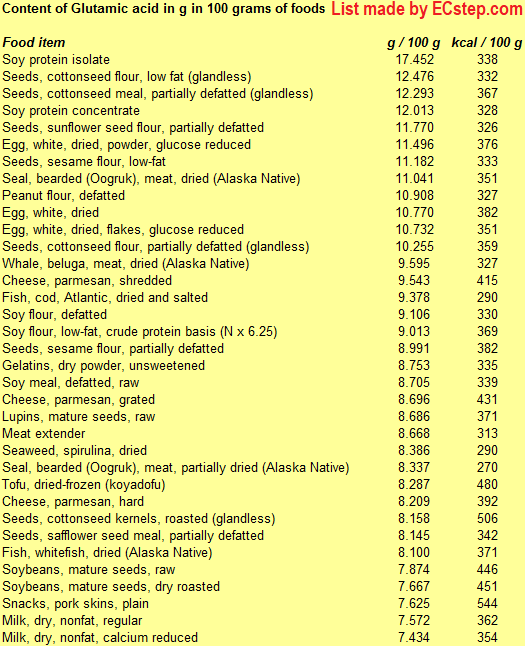
Reference 1
Glutamine
Glutamine (symbol Gln) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, polar amino acid. It is conditionally essential in humans, meaning the body can usually synthesize sufficient amounts of it, but in some instances of stress, the body’s demand for glutamine increases, and glutamine must be obtained from the diet.
In human blood, glutamine is the most abundant free amino acid.
Sources
The dietary sources of glutamine includes especially the protein-rich foods like beef, chicken, fish, dairy products, eggs, vegetables like beans, beets, cabbage, spinach, carrots, parsley, vegetable juices and also in wheat, papaya, Brussels sprouts, celery, kale and fermented foods like miso. See also table below.
Functions
Glutamine plays a role in a variety of biochemical functions:
- Protein synthesis, as any other of the 20 proteinogenic amino acids
- Lipid synthesis, especially by cancer cells
- Regulation of acid-base balance in the kidney by producing ammonium
- Cellular energy, as a source, next to glucose
- Nitrogen donation for many anabolic processes, including the synthesis of purines
- Carbon donation, as a source, refilling the citric acid cycle
- Nontoxic transporter of ammonia in the blood circulation
- Precursor to the neurotransmitter glutamate
On the level of tissue, glutamine plays a role in maintaining the normal integrity of the intestinal mucosa, but randomised trials provide no evidence of any benefit of nutritional supplementation.
Producers
Glutamine is synthesized by the enzyme glutamine synthetase from glutamate and ammonia. The most relevant glutamine-producing tissue is the muscle mass, accounting for about 90% of all glutamine synthesized.
Glutamine is also released, in small amounts, by the lungs and brain. Although the liver is capable of relevant glutamine synthesis, its role in glutamine metabolism is more regulatory than producing, since the liver takes up large amounts of glutamine derived from the gut.
Consumers
The most eager consumers of glutamine are the cells of intestines, the kidney cells for the acid-base balance, activated immune cells, and many cancer cells.
Uses
Nutrition
Glutamine is the most abundant naturally occurring, nonessential amino acid in the human body, and one of the few amino acids that can directly cross the blood–brain barrier. Humans obtain glutamine through catabolism of proteins in foods they eat. In states where tissue is being built or repaired, like growth of babies, or healing from wounds or severe illness, glutamine becomes conditionally essential.
Medical food
Glutamine is marketed as medical food and is prescribed when a medical professional believes a person in their care needs supplementary glutamine due to metabolic demands beyond what can be met by endogenous synthesis or diet.
Safety
Glutamine is safe in adults and in preterm infants. Although glutamine is metabolized to glutamate and ammonia, both of which have neurological effects, their concentrations are not increased much, and no adverse neurological effects were detected. The observed safe level for supplemental L-glutamine in normal healthy adults is 14 g/day.
Adverse effects of glutamine have been described for people receiving home parenteral nutrition and those with liver-function abnormalities. Although glutamine has no effect on the proliferation of tumor cells, it is still possible that glutamine supplementation may be detrimental in some cancer types.
Ceasing glutamine supplementation in people adapted to very high consumption may initiate a withdrawal effect, raising the risk of health problems such as infections or impaired integrity of the intestine.
Research
Glutamine mouthwash may be useful to prevent oral mucositis in people undergoing chemotherapy but intravenous glutamine does not appear useful to prevent mucositis in the GI tract.
Glutamine supplementation was thought to have potential to reduce complications in people who are critically ill or who have had abdominal surgery but this was based on poor quality clinical trials.
Supplementation does not appear to be useful in adults or children with Crohn’s disease or inflammatory bowel disease, but clinical studies as of 2016 were underpowered. Supplementation does not appear to have an effect in infants with significant problems of the stomach or intestines.
–
Below is a list of foods having the highest content of glutamic acid and glutamine taken together (since the acid forms are easily converted to the amide forms and vice versa) in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.

Reference 1
Glycine
Glycine (symbol Gly) is the amino acid that has a single hydrogen atom as its side chain. It is the simplest possible amino acid. Glycine is one of the proteinogenic amino acids. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. The acyl radical is glycyl.
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phosphoglycerate, but the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis.
Degradation
Glycine is degraded via three pathways. The predominant pathway in animals and plants is the reverse of the glycine synthase pathway mentioned above.
The half-life of glycine and its elimination from the body varies significantly based on dose. In one study, the half-life varied between 0.5 and 4.0 hours.
Physiological function
The principal function of glycine is as a precursor to proteins. Most proteins incorporate only small quantities of glycine, a notable exception being collagen, which contains about 35% glycine due to its periodically repeated role in the formation of collagen’s helix structure in conjunction with hydroxyproline.
A biosynthetic intermediate
In higher eukaryotes, δ-aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA by the enzyme ALA synthase. Glycine provides the central C2N subunit of all purines.
A neurotransmitter
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potential (IPSP).
Animal and human foods
USP glycine has a wide variety of uses, including as an additive in pet food and animal feed, in foods and pharmaceuticals as a sweetener/taste enhancer, or as a component of food supplements and protein drinks.
Cosmetics and miscellaneous applications
Glycine serves as a buffering agent in antacids, analgesics, antiperspirants, cosmetics, and toiletries.
A variety of industrial and chemical processes use glycine or its derivatives, such as the production of fertilizers and metal complexing agents.
Presence in space
The presence of glycine outside the earth was confirmed in 2009, based on the analysis of samples that had been taken in 2004 by the NASA spacecraft Stardust from comet Wild 2 and subsequently returned to earth. Glycine had previously been identified in the Murchison meteorite in 1970. The discovery of cometary glycine bolstered the theory of panspermia, which claims that the “building blocks” of life are widespread throughout the Universe.
–
Below is a list of foods having the highest content of glycine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
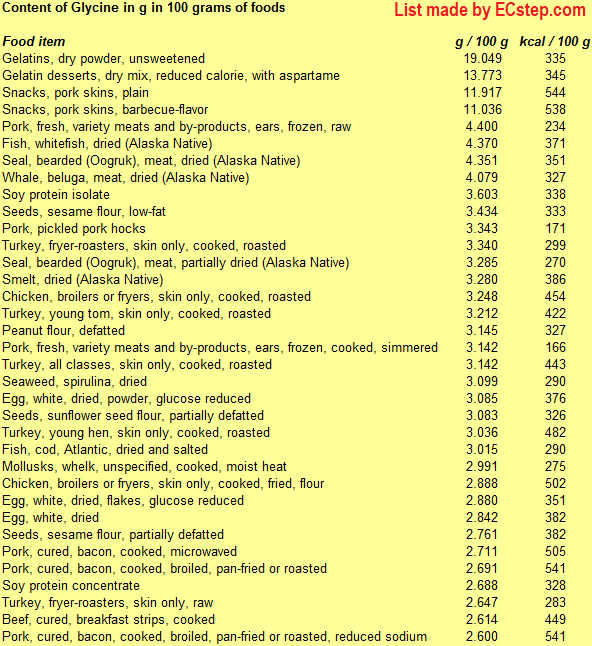
Reference 1
Histidine
Histidine (symbol His) is an α-amino acid that is used in the biosynthesis of proteins. Initially thought essential only for infants, longer-term studies have shown it is essential for adults also.
It is a precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical is histidyl.
The imidazole sidechain of histidine is a common coordinating ligand in metalloproteins and is a part of catalytic sites in certain enzymes.
In carbonic anhydrases, a histidine proton shuttle is utilized to rapidly shuttle protons away from a zinc-bound water molecule to quickly regenerate the active form of the enzyme.
Histidine is also important in haemoglobin in helices E and F. Histidine assists in stabilising oxyhaemoglobin and destabilising CO-bound haemoglobin. As a result, carbon monoxide binding is only 200 times stronger in haemoglobin, compared to 20,000 times stronger in free haem.
Histidine is synthesized from phosphoribosyl pyrophosphate (PRPP), a biochemical intermediate, which is made from ribose-5-phosphate by ribose-phosphate diphosphokinase during the pentose phosphate pathway.
Degradation
Histidine is one of the amino acids that can be converted to intermediates of the tricarboxylic acid (TCA) cycle.
Histidine, along with other amino acids such as proline and arginine, takes part in deamination, a process in which its amino group is removed.
Overall, the degradation results in the formation of glutamate and ammonia.
Glutamate can then be deaminated by glutamate dehydrogenase or transaminated to form α-ketoglutarate.
Histidine is a precursor for histamine, an amine produced in the body necessary for inflammation.
Histidine can be converted to 3-methylhistidine, which serves as a biomarker for skeletal muscle damage, by certain methyltransferase enzymes.
Histidine is also a precursor for carnosine biosynthesis, which is a dipeptide found in skeletal muscle.
Requirements
The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002. For histidine, for adults 19 years and older, 14 mg/kg body weight/day.
–
Below is a list of foods having the highest content of histidine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
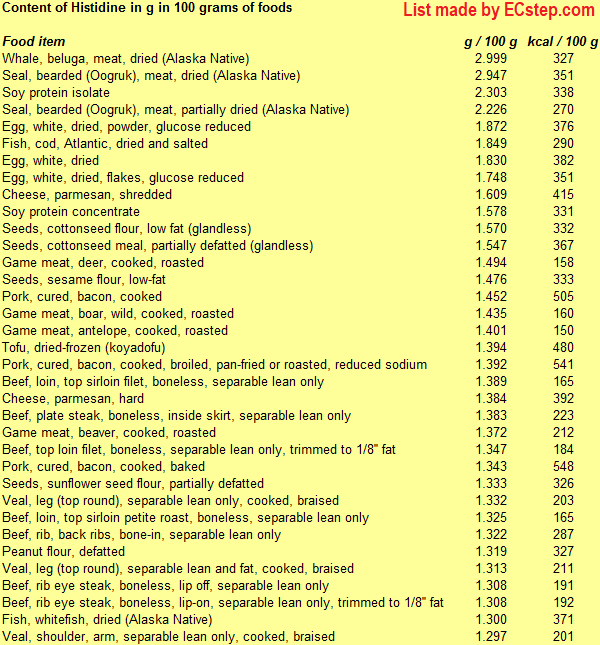
Reference 1
Isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet.
Isoleucine, like other branched-chain amino acids, is associated with insulin resistance: higher levels of isoleucine are observed in the blood of diabetic humans.
Mice fed a isoleucine deprivation diet for one day have improved insulin sensitivity, and feeding of a isoleucine deprivation diet for one week significantly decreases blood glucose levels.
In diet-induced obese and insulin resistant mice, a diet with decreased levels of isoleucine and the other branched-chain amino acids results in reduced adiposity and improved insulin sensitivity.
In humans, a protein restricted diet lowers blood levels of isoleucine and decreases fasting blood glucose levels.
–
Below is a list of foods having the highest content of isoleucine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
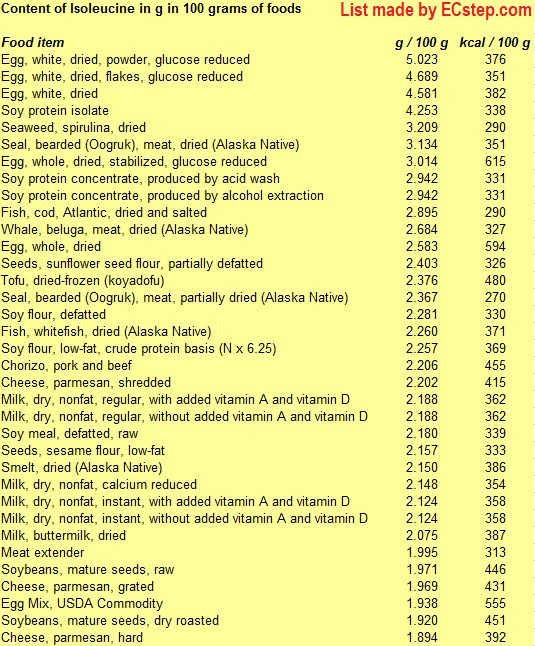
Reference 1
Leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet.
Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. See also table below.
Like valine and isoleucine, leucine is a branched-chain amino acid. The primary metabolic end products of leucine metabolism are acetyl-CoA and acetoacetate; consequently, it is one of the two exclusively ketogenic amino acids, with lysine being the other. It is the most important ketogenic amino acid in humans.
Leucine and β-hydroxy β-methylbutyric acid, a minor leucine metabolite, exhibit pharmacological activity in humans and have been demonstrated to promote protein biosynthesis via the phosphorylation of the mechanistic target of rapamycin (mTOR).
As a dietary supplement, leucine has been found to slow the degradation of muscle tissue by increasing the synthesis of muscle proteins in aged rats. However, results of comparative studies are conflicting.
Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men, but more studies are needed.
Both L-leucine and D-leucine protect mice against seizures. D-leucine also terminates seizures in mice after the onset of seizure activity, at least as effectively as diazepam and without sedative effects. Decreased dietary intake of L-leucine promotes adiposity in mice.
High blood levels of leucine are associated with insulin resistance in humans, mice, and rodents. Dietary restriction of leucine and the other BCAAs can reverse diet-induced obesity in wild-type mice by increasing energy expenditure, and can restrict fat mass gain of hyperphagic rats.
A high intake of leucine may cause or exacerbate symptoms of pellagra in people with low niacin status because it interferes with the conversion of L-tryptophan to niacin.
Leucine at a dose exceeding 500 mg/kg/d was observed with hyperammonemia. As such, unofficially, a tolerable upper intake level (UL) for leucine in healthy adult men can be suggested at 500 mg/kg/d under acute dietary conditions.
–
Below is a list of foods having the highest content of leucine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
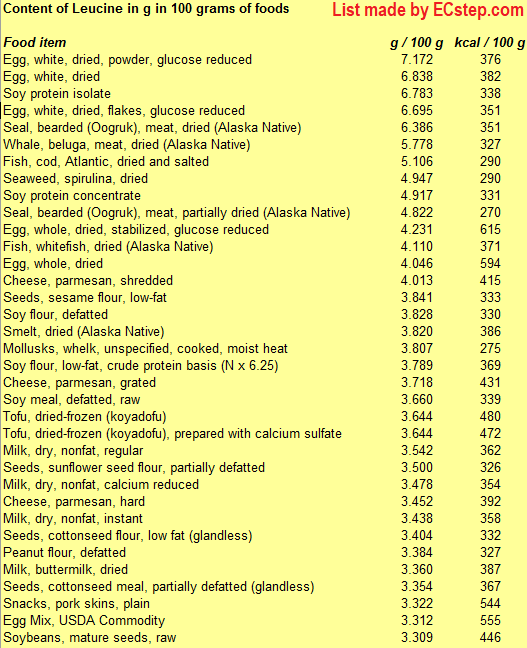
Reference 1
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is used in the biosynthesis of proteins.
The human body cannot synthesize lysine, so it is essential in humans and must be obtained from the diet. Lysine catabolism occurs through one of several pathways, the most common of which is the saccharopine pathway.
Lysine plays several roles in humans, most importantly proteinogenesis, but also in the crosslinking of collagen polypeptides, uptake of essential mineral nutrients, and in the production of carnitine, which is key in fatty acid metabolism. Lysine is also often involved in histone modifications, and thus, impacts the epigenome
Due to its importance in several biological processes, a lack of lysine can lead to several disease states including defective connective tissues, impaired fatty acid metabolism, anaemia, and systemic protein-energy deficiency. In contrast, an overabundance of lysine, caused by ineffective catabolism, can cause severe neurological issues.
Lysine is one of the nine essential amino acids in humans. The daily human nutritional requirements varies from ~60 mg/kg in infancy to ~30 mg/kg in adults.
This requirement is commonly met in a western society with the intake of lysine from meat and vegetable sources well in excess of the recommended requirement. See also table below.
In vegetarian diets, the intake of lysine is less due to the limiting quantity of lysine in cereal crops compared to meat sources. Plants accumulate lysine and other amino acids in the form of seed storage proteins, found within the seeds of the plant, and this represents the edible component of cereal crops.
Commonly, to overcome the limiting abundance of lysine in livestock feed, industrially produced lysine is added. The industrial process includes the fermentative culturing of Corynebacterium glutamicum and the subsequent purification of lysine.
Biological roles
The most common role for lysine is proteinogenesis. Lysine frequently plays an important role in protein structure. Lysine can be found buried as well as more commonly in solvent channels and on the exterior of proteins, where it can interact with the aqueous environment. Lysine can also contribute to protein stability.
A second major role of lysine is in epigenetic regulation by means of histone modification. There are several types of covalent histone modifications, which commonly involve lysine residues found in the protruding tail of histones. The various modifications have downstream effects on gene regulation, in which genes can be activated or repressed.
Lysine has also been implicated to play a key role in other biological processes including: structural proteins of connective tissues, calcium homeostasis, and fatty acid metabolism.
Lysine has been shown to be involved in the crosslinking between the three helical polypeptides in collagen, resulting in its stability and tensile strength.
Lysine has also been proposed to be involved in calcium intestinal absorption and renal retention, and thus, may play a role in calcium homeostasis.
The role of lysine in collagen has been outlined above, however, a lack of lysine and hydroxylysine involved in the crosslinking of collagen peptides has been linked to a disease state of the connective tissue.
Lysine has also been shown to play a role in anaemia, as lysine is suspected to have an effect on the uptake of iron and, subsequently, the concentration of ferritin in blood plasma. However, the exact mechanism of action is yet to be elucidated.
–
Below is a list of foods having the highest content of lysine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
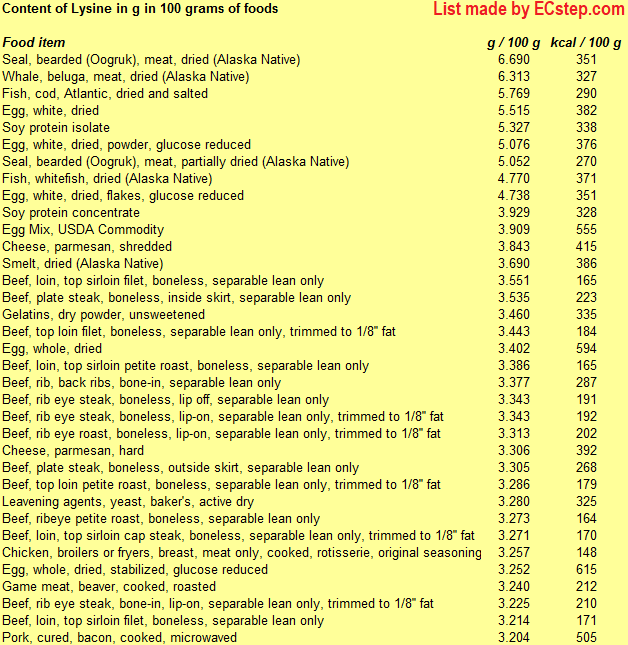
Reference 1
Methionine
Methionine (symbol Met) is an essential amino acid in humans. As the substrate for other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans.
Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, being the methyl group donor in DNA methylation, is related to cancer growth in a number of studies
Requirements
The Food and Nutrition Board of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002. For methionine combined with cysteine the RDA for adults 19 years and older is 19 mg/kg body weight/day.
Dietary sources
Good food sources of Methionine are egg white, dried, sesame seeds flour, whole eggs, parmesan cheese, Brazil nuts and soy protein concentrate.
High levels of methionine can be found in eggs, meat, and fish; sesame seeds, Brazil nuts, and cereal grains. Most fruits and vegetables contain very little. Most legumes, though protein dense, are low in methionine. See also table below.
Proteins without adequate methionine are not considered to be complete proteins. For that reason, methionine is sometimes added as an ingredient to pet foods.
Restriction
Some scientific evidence indicates restricting methionine consumption can increase lifespans in fruit flies.
A 2005 study showed methionine restriction without energy restriction extends mouse lifespans. This extension requires intact growth hormone signaling, as animals without intact growth-hormone signaling do not have a further increase in lifespan when methionine restricted. The metabolic response to methionine restriction is also altered in mouse growth hormone signaling mutants.
A study published in Nature showed adding just the essential amino acid methionine to the diet of fruit flies under dietary restriction, including restriction of essential amino acids (EAAs), restored fertility without reducing the longer lifespans that are typical of dietary restriction, leading the researchers to determine that methionine “acts in combination with one or more other EAAs to shorten lifespan.”
Restoring methionine to the diet of mice on a dietary restriction regimen blocks many acute benefits of dietary restriction, a process that may be mediated by increased production of hydrogen sulfide.
Several studies showed that methionine restriction also inhibits aging-related disease processes in mice and inhibits colon carcinogenesis in rats.
In humans, methionine restriction through dietary modification could be achieved through a vegan diet. Veganism being a completely plant based diet is typically very low in methionine, but certain nuts and legumes may provide higher levels.
A 2009 study on rats showed “methionine supplementation in the diet specifically increases mitochondrial ROS (reactive oxygen species) production and mitochondrial DNA oxidative damage in rat liver mitochondria offering a plausible mechanism for its hepatotoxicity”.
However, since methionine is an essential amino acid, it cannot be entirely removed from animals’ diets without disease or death occurring over time.
For example, rats fed a diet without methionine and choline developed steatohepatitis (fatty liver) and anemia, and lost two-thirds of their body weight over 5 weeks.
Administration of methionine ameliorated the pathological consequences of methionine deprivation.
Short-term removal of only methionine from the diet can reverse diet-induced obesity and promotes insulin sensitivity in mice.
Health
Loss of methionine has been linked to senile greying of hair. Its lack leads to a buildup of hydrogen peroxide in hair follicles, a reduction in tyrosinase effectiveness, and a gradual loss of hair color.
Methionine is an intermediate in the biosynthesis of cysteine, carnitine, taurine, lecithin, phosphatidylcholine, and other phospholipids.
Improper conversion of methionine can lead to atherosclerosis due to accumulation of homocysteine.
Methionine might also be essential to reversing damaging methylation of glucocorticoid receptors caused by repeated stress exposures, with implications for depression.
–
Below is a list of foods having the highest content of methionine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
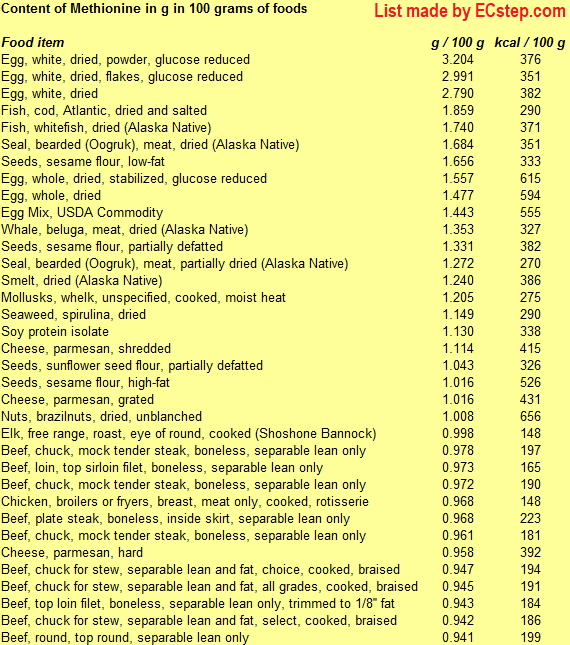
Reference 1
Phenylalanine
Phenylalanine (symbol Phe) is an essential α-amino acid.
It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine.
The L-isomer is used to biochemically form proteins, coded for by DNA.
Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the skin pigment melanin.
Phenylalanine is found naturally in the breast milk of mammals.
It is used in the manufacture of food and drink products and sold as a nutritional supplement for its reputed analgesic and antidepressant effects.
It is a direct precursor to the neuromodulator phenethylamine, a commonly used dietary supplement.
As an essential amino acid, phenylalanine is not synthesized de novo in humans and other animals, who must ingest phenylalanine or phenylalanine-containing proteins.
Dietary sources
Good sources of phenylalanine are eggs, chicken, liver, beef, milk, and soybeans. See also table below.
Dietary recommendations
The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002. For phenylalanine plus tyrosine RDA for adults 19 years and older is 33 mg/kg body weight/day.
Other biological roles
L-Phenylalanine is biologically converted into L-tyrosine, another one of the DNA-encoded amino acids. L-tyrosine in turn is converted into L-DOPA, which is further converted into the catecholamines dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline).
Phenylalanine uses the same active transport channel as tryptophan to cross the blood–brain barrier. In excessive quantities, supplementation can interfere with the production of serotonin and other aromatic amino acids as well as nitric oxide due to the overuse (eventually, limited availability) of the associated cofactors, iron or tetrahydrobiopterin.
The corresponding enzymes in for those compounds are the aromatic amino acid hydroxylase family and nitric oxide synthase.
The genetic disorder phenylketonuria (PKU) is the inability to metabolize phenylalanine because of a lack of the enzyme phenylalanine hydroxylase. Individuals with this disorder are known as “phenylketonurics” and must regulate their intake of phenylalanine.
A non-food source of phenylalanine is the artificial sweetener aspartame. This compound is metabolized by the body into several chemical byproducts including phenylalanine. The breakdown problems phenylketonurics have with the buildup of phenylalanine in the body also occurs with the ingestion of aspartame, although to a lesser degree.
–
Below is a list of foods having the highest content of phenylanaline in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
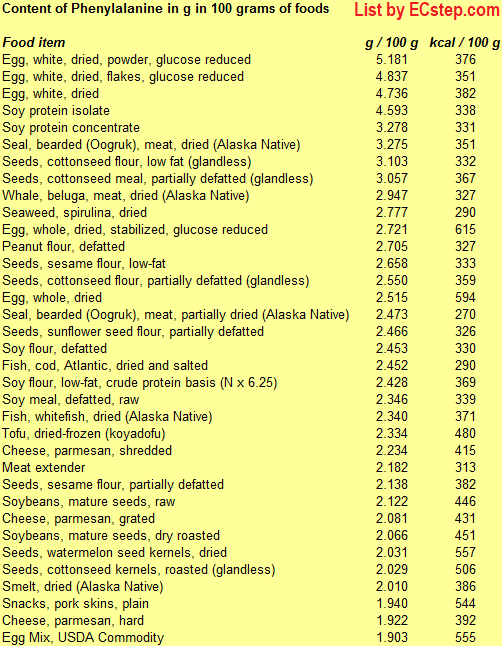
Reference 1
Proline
Proline (symbol Pro) is a proteinogenic amino acid that is used in the biosynthesis of proteins. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate.
Proline is biosynthetically derived from the amino acid L-glutamate.
Biological activity
L-Proline has been found to act as a weak agonist of the glycine receptor and of both NMDA and non-NMDA (AMPA/kainate) ionotropic glutamate receptors. It has been proposed to be a potential endogenous excitotoxin.
Properties in protein structure
The exceptional conformational rigidity of proline affects the secondary structure of proteins near a proline residue and may account for proline’s higher prevalence in the proteins of thermophilic organisms.
Multiple prolines and/or hydroxyprolines in a row can create a polyproline helix, the predominant secondary structure in collagen. The hydroxylation of proline by prolyl hydroxylase (or other additions of electron-withdrawing substituents such as fluorine) increases the conformational stability of collagen significantly.
Hence, the hydroxylation of proline is a critical biochemical process for maintaining the connective tissue of higher organisms. Severe diseases such as scurvy can result from defects in this hydroxylation, e.g., mutations in the enzyme prolyl hydroxylase or lack of the necessary ascorbate (vitamin C) cofactor.
Uses
In brewing, proteins rich in proline combine with polyphenols to produce haze (turbidity).
L-Proline is an osmoprotectant and therefore is used in many pharmaceutical, biotechnological applications.
–
Below is a list of foods having the highest content of proline in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
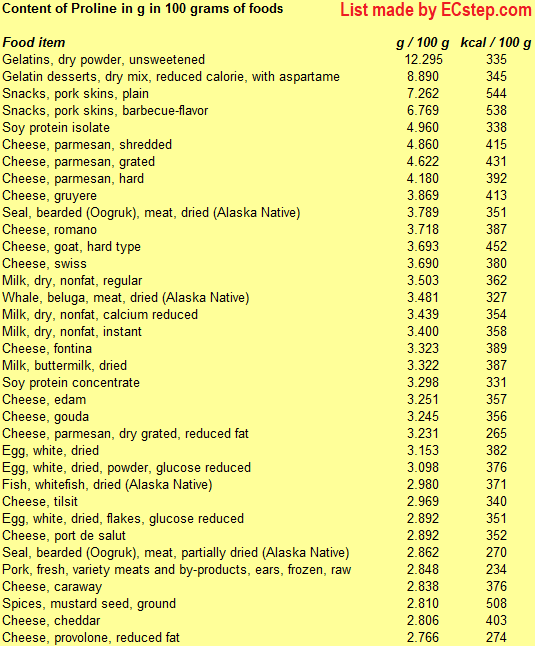
Reference 1
Serine
Serine (symbol Ser) is an ɑ-amino acid that is used in the biosynthesis of proteins. It can be synthesized in the human body under normal physiological circumstances, making it a non-essential amino acid.
This compound is one of the naturally occurring proteinogenic amino acids. It is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source. Its name is derived from the Latin for silk, sericum.
Serine is important in metabolism in that it participates in the biosynthesis of purines and pyrimidines. It is the precursor to several amino acids including glycine and cysteine, as well as tryptophan in bacteria. It is also the precursor to numerous other metabolites, including sphingolipids and folate.
Structural role
Serine plays an important role in the catalytic function of many enzymes. It has been shown to occur in the active sites of chymotrypsin, trypsin, and many other enzymes.
The so-called nerve gases and many substances used in insecticides have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely.
As a constituent (residue) of proteins, its side chain can undergo O-linked glycosylation, which may be functionally related to diabetes.
It is one of three amino acid residues that are commonly phosphorylated by kinases during cell signaling in eukaryotes.
D-serine was thought to exist only in bacteria until relatively recently; it was the second D amino acid discovered to naturally exist in humans, present as a signaling molecule in the brain, soon after the discovery of D-aspartate.
Had D amino acids been discovered in humans sooner, the glycine site on the NMDA receptor might instead be named the D-serine site.
Apart from central nervous system, D-serine plays a signaling role in peripheral tissues and organs such as cartilage, kidney and corpus cavernosum.
Gustatory sensation
L-Serine is sweet with minor umami and sour tastes at high concentration.
Pure D-serine is an off-white crystalline powder with a very faint musty aroma. D-Serine is sweet with an additional minor sour taste at medium and high concentrations.
Research for therapeutic use
D-Serine is being studied in rodents as a potential treatment for schizophrenia and L-serine is in a FDA-approved human clinical trial as a possible treatment for Amyotrophic Lateral Sclerosis (ALS).
D-Serine has also been described as a potential biomarker for early Alzheimer’s disease (AD) diagnosis, due to a relatively high concentration of it in the cerebrospinal fluid of probable AD patients.
–
Below is a list of foods having the highest content of serine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
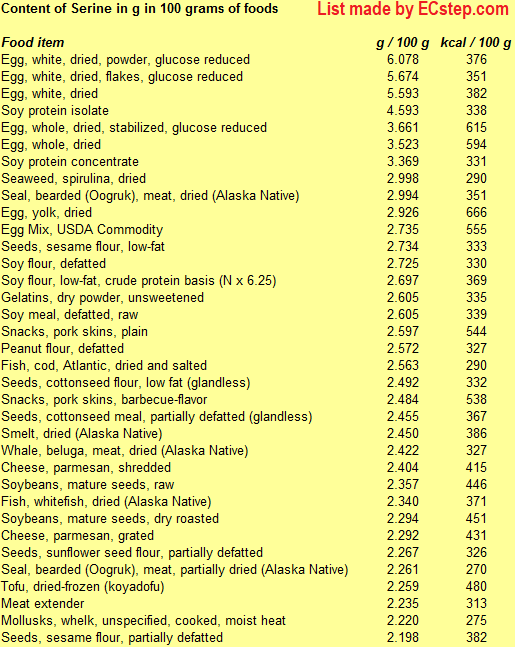
References 1
Threonine
Threonine (symbol Thr) is an amino acid that is used in the biosynthesis of proteins.
It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet.
Threonine needs to be present in proteins in the diet. Adult humans require about 20 mg/kg body weight/day.
Metabolism
Threonine is metabolized in two ways:
In many animals it is converted to pyruvate via threonine dehydrogenase. An intermediate in this pathway can undergo thiolysis with CoA to produce acetyl-CoA and glycine.
In humans threonine is converted to α-ketobutyrate.
Sources
Foods high in threonine include cottage cheese, poultry, fish, meat, lentils, Black turtle bean and Sesame seeds.
Below is a list of foods having the highest content of threonine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
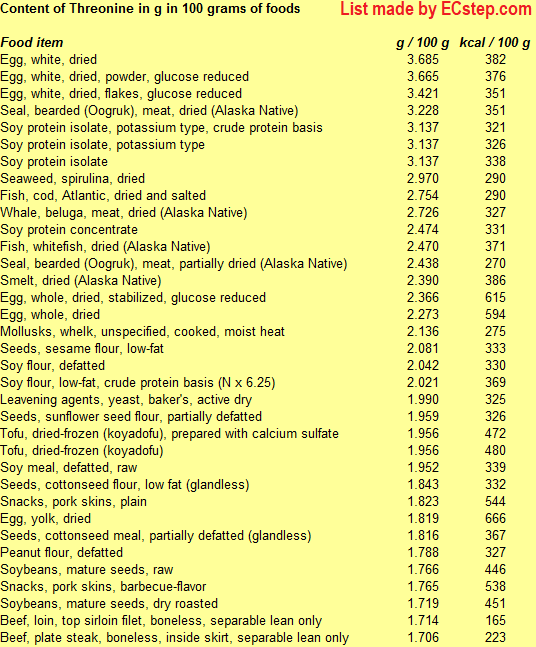
Reference 1
Tryptophan
Tryptophan (symbol Trp) is an α-amino acid that is used in the biosynthesis of proteins. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet.
Tryptophan is also a precursor to the neurotransmitter serotonin and the hormone melatonin.
Function
Amino acids, including tryptophan, are used as building blocks in protein biosynthesis, and proteins are required to sustain life.
Tryptophan is among the less common amino acids found in proteins, but it plays important structural or functional roles whenever it occurs.
For instance, tryptophan and tyrosine residues play special roles in “anchoring” membrane proteins within the cell membrane.
In addition, tryptophan functions as a biochemical precursor for the following compounds:
Serotonin (a neurotransmitter), synthesized by tryptophan hydroxylase.
Melatonin (a neurohormone) is in turn synthesized from serotonin..
Niacin, also known as vitamin B3, is synthesized from tryptophan.
Auxins (a class of phytohormones) are synthesized from tryptophan.
The disorder fructose malabsorption causes improper absorption of tryptophan in the intestine, reduced levels of tryptophan in the blood, and depression.
Recommended dietary allowance
The RDA of tryptophan is 5 mg/kg body weight/day for adults 19 years and over.
Dietary sources
Tryptophan is present in most protein-based foods or dietary proteins. It is particularly plentiful in chocolate, oats, dried dates, milk, yogurt, cottage cheese, red meat, eggs, fish, poultry, sesame, chickpeas, almonds, sunflower seeds, pumpkin seeds, buckwheat, spirulina, and peanuts. See also table below.
Use as a dietary supplement
Because tryptophan is converted into 5-hydroxytryptophan (5-HTP) which is then converted into the neurotransmitter serotonin, it has been proposed that consumption of tryptophan or 5-HTP may improve depression symptoms by increasing the level of serotonin in the brain.
Tryptophan is sold over the counter in the United States and the United Kingdom as a dietary supplement for use as an antidepressant, anxiolytic, and sleep aid.
It is also marketed as a prescription drug in some European countries for the treatment of major depression.
There is evidence that blood tryptophan levels are unlikely to be altered by changing the diet, but consuming purified tryptophan increases the serotonin level in the brain, whereas eating foods containing tryptophan does not.
This is because the transport system that brings tryptophan across the blood–brain barrier also transports other amino acids which are contained in protein food sources.
High blood plasma levels of other large neutral amino acids prevent the plasma concentration of tryptophan from increasing brain concentration levels.
In 2001 a Cochrane review of the effect of 5-HTP and tryptophan on depression was published. The authors included only studies of a high rigor and included both 5-HTP and tryptophan in their review because of the limited data on either. Of 108 studies of 5-HTP and tryptophan on depression published between 1966 and 2000, only two met the authors’ quality standards for inclusion, totaling 64 study participants.
The substances were more effective than placebo in the two studies included but the authors state that “the evidence was of insufficient quality to be conclusive” and note that “because alternative antidepressants exist which have been proven to be effective and safe, the clinical usefulness of 5-HTP and tryptophan is limited at present”.
The use of tryptophan as an adjunctive therapy in addition to standard treatment for mood and anxiety disorders is not supported by the scientific evidence.
Side effects
Potential side effects of tryptophan supplementation include nausea, diarrhea, drowsiness, lightheadedness, headache, dry mouth, blurred vision, sedation, euphoria, and nystagmus (involuntary eye movements).
Interactions
Tryptophan taken as a dietary supplement (such as in tablet form) has the potential to cause serotonin syndrome when combined with antidepressants of the MAOI or SSRI class or other strongly serotonergic drugs. Because tryptophan supplementation has not been thoroughly studied in a clinical setting, its interactions with other drugs are not well known.
Eosinophilia–myalgia syndrome
There was a large outbreak of eosinophilia-myalgia syndrome (EMS) in the U.S. in 1989, with more than 1,500 cases reported to the CDC and at least 37 deaths.
After preliminary investigation revealed that the outbreak was linked to intake of tryptophan, the U.S. Food and Drug Administration (FDA) recalled tryptophan supplements in 1989 and banned most public sales in 1990, with other countries following suit.
Other evidence suggests that tryptophan itself may be a potentially major contributory factor in EMS.
The FDA loosened its restrictions on sales and marketing of tryptophan in February 2001, but continued to limit the importation of tryptophan not intended for an exempted use until 2005.
Turkey meat and drowsiness
A common assertion in the US is that heavy consumption of turkey meat results in drowsiness, due to high levels of tryptophan contained in turkey. However, the amount of tryptophan in turkey is comparable to that contained in other meats.
Drowsiness after eating may be caused by other foods eaten with the turkey, particularly carbohydrates. Ingestion of a meal rich in carbohydrates triggers the release of insulin. Insulin in turn stimulates the uptake of large neutral branched-chain amino acids (BCAA), but not tryptophan, into muscle, increasing the ratio of tryptophan to BCAA in the blood stream.
The resulting increased tryptophan ratio reduces competition at the large neutral amino acid transporter (which transports both BCAA and aromatic amino acids), resulting in more uptake of tryptophan across the blood–brain barrier into the cerebrospinal fluid (CSF).
Once in the CSF, tryptophan is converted into serotonin in the raphe nuclei by the normal enzymatic pathway. The resultant serotonin is further metabolised into melatonin by the pineal gland. Hence, these data suggest that “feast-induced drowsiness”—or postprandial somnolence—may be the result of a heavy meal rich in carbohydrates, which indirectly increases the production of melatonin in the brain, and thereby promotes sleep.
Research
Tryptophan affects brain serotonin synthesis when given orally in a purified form. Low brain serotonin level is induced by administration of tryptophan-poor protein in a technique called “acute tryptophan depletion”.
Studies using this method have evaluated the effect of serotonin on mood and social behavior, finding that serotonin reduces aggression and increases agreeableness.
–
Below is a list of foods having the highest content of tryptophan in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
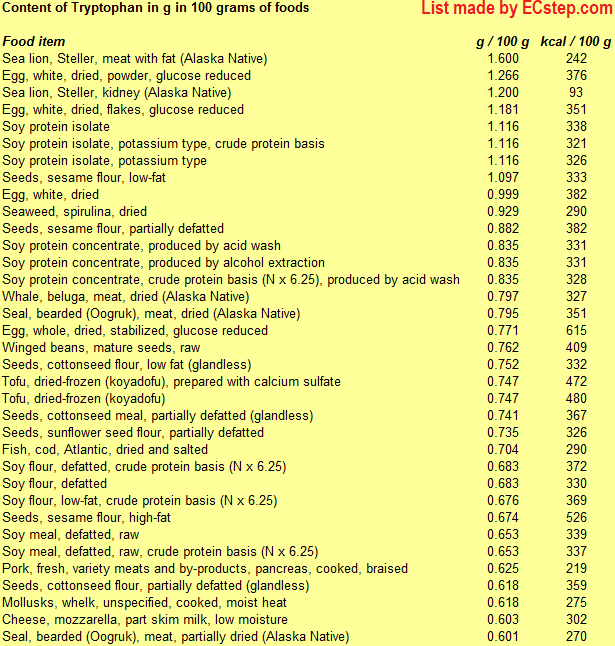
Reference 1
Tyrosine
Tyrosine (symbol Tyr) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word “tyrosine” is from the Greek tyros, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese.
Functions
Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes. It functions as a receiver of phosphate groups that are transferred by way of protein kinases.
Dietary requirements and sources
The Dietary Reference Intake (recommended dietary allowance, RDA) for phenylalanine and tyrosine is 33 mg per kilogram of body weight. For a 70 kg person, this is 2310 mg (phenylalanine + tyrosine).
Tyrosine, which can also be synthesized in the body from phenylalanine, is found in many high-protein food products such as chicken, turkey, fish, milk, yogurt, cottage cheese, cheese, peanuts, almonds, pumpkin seeds, sesame seeds, soy products, lima beans, avocados, and bananas. For example, the white of an egg has about 250 mg per egg, while lean beef/lamb/pork/salmon/chicken/turkey contains about 1000 mg per 3 ounces (85 g) portion. See also table below.
Biosynthesis
Mammals synthesize tyrosine from the essential amino acid phenylalanine (phe), which is derived from food. The conversion of phe to tyr is catalyzed by the enzyme phenylalanine hydroxylase.
Precursor to neurotransmitters and hormones
In dopaminergic cells in the brain, tyrosine is converted to L-DOPA by the enzyme tyrosine hydroxylase (TH). TH is the rate-limiting enzyme involved in the synthesis of the neurotransmitter dopamine. Dopamine can then be converted into other catecholamines, such as norepinephrine (noradrenaline) and epinephrine (adrenaline).
The thyroid hormones triiodothyronine (T3) and thyroxine (T4) in the colloid of the thyroid also are derived from tyrosine.
Precursor to pigments
Tyrosine is the precursor to the pigment melanin.
Role in coenzyme Q10 synthesis
Tyrosine (or its precursor phenylalanine) is needed to synthesize the benzoquinone structure which forms part of coenzyme Q10.
Degradation
The decomposition of tyrosine to acetoacetate and fumarate. Two dioxygenases are necessary for the decomposition path. The end products can then enter into the citric acid cycle.
m-Tyrosine and analogues (rare in nature but available synthetically) have shown application in Parkinson’s Disease, Alzheimer’s disease and arthritis.
Medical use
Tyrosine is a precursor to neurotransmitters and increases plasma neurotransmitter levels (particularly dopamine and norepinephrine), but has little if any effect on mood in normal subjects. The effect on mood is noted in humans subjected to stressful conditions (see below).
A number of studies have found tyrosine to be useful during conditions of stress, cold, fatigue, prolonged work and sleep deprivation, with reductions in stress hormone levels, reductions in stress-induced weight loss seen in animal trials, and improvements in cognitive and physical performance seen in human trials.
Tyrosine does not seem to have any significant effect on cognitive or physical performance in normal circumstances, but does help sustain working memory better during multitasking.
–
Below is a list of foods having the highest content of tyrosine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
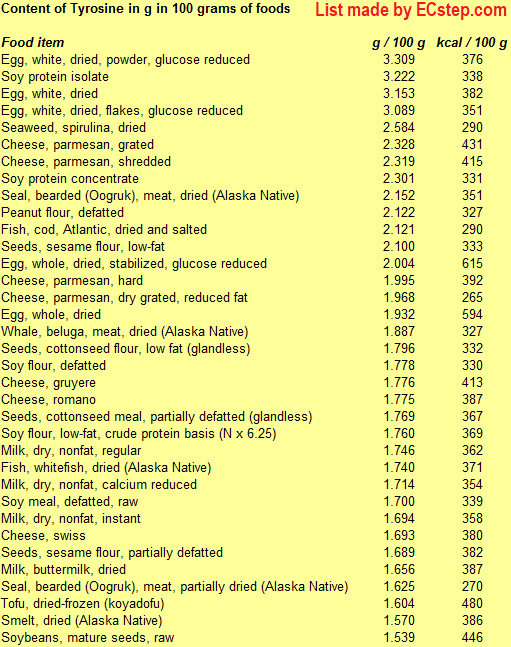
Reference 1
Valine
Valine (symbol Val) is an α-amino acid that is used in the biosynthesis of proteins. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet.
Like leucine and isoleucine, valine is a branched-chain amino acid. In sickle-cell disease, a single glutamic acid in β-globin is replaced with valine. Because valine is hydrophobic, whereas glutamic acid is hydrophilic, this change makes the hemoglobin prone to abnormal aggregation.
Requirement
Adult humans require about 4 mg/kg body weight daily.
Sources
Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, beans and legumes. See also table below.
Degradation
Like other branched-chain amino acids, the catabolism of valine starts with the removal of the amino group by transamination, giving alpha-ketoisovalerate, an alpha-keto acid, which is converted to isobutyryl-CoA through oxidative decarboxylation by the branched-chain α-ketoacid dehydrogenase complex.
This is further oxidised and rearranged to succinyl-CoA, which can enter the citric acid cycle.
Valine, like other branched-chain amino acids, is associated with insulin resistance: higher levels of valine are observed in the blood of diabetic humans. Mice fed a valine deprivation diet for one day have improved insulin sensitivity, and feeding of a valine deprivation diet for one week significantly decreases blood glucose levels.
In diet-induced obese and insulin resistant mice, a diet with decreased levels of valine and the other branched-chain amino acids results in reduced adiposity and improved insulin sensitivity.
The valine catabolite 3-hydroxyisobutyrate promotes skeletal muscle insulin resistance in mice by stimulating fatty acid uptake into muscle and lipid accumulation. In humans, a protein restricted diet lowers blood levels of valine and decreases fasting blood glucose levels.
Hematopoietic stem cells
Dietary valine is essential for hematopoietic stem cell (HSC) self-renewal, as demonstrated by experiments in mice. Dietary valine restriction selectively depletes long-term repopulating HSC in mouse bone marrow.
Successful stem cell transplantation was achieved in mice without irradiation after 3 weeks on a valine restricted diet. Long term survival of the transplanted mice was achieved when valine was returned to the diet gradually over a 2-week period to avoid refeeding syndrome.
–
Below is a list of foods having the highest content of valine in g per 100 grams of food.
Since you may also be interested in foods with a high amino acid content AND few calories, the list also includes the number of calories.
The list is made by ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
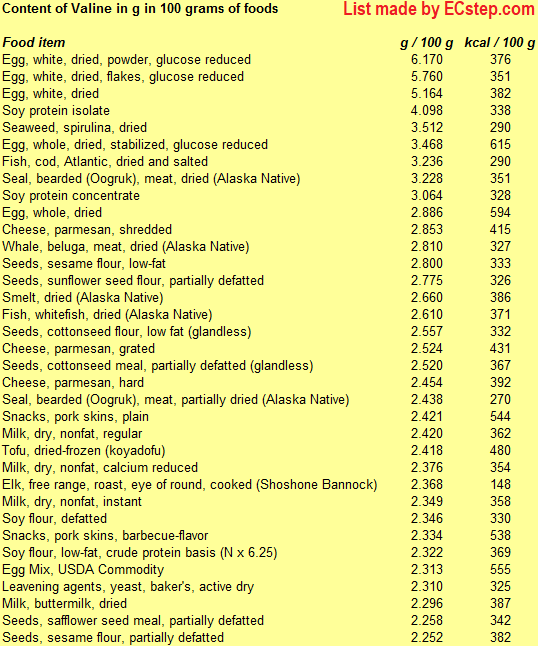
Reference 1


