Protein
 Protein is the most important component of the human body. Protein is present in all cells making up the enzymes and structural components (tendons, ligaments), contractile proteins (muscle, including the heart), antibodies, hormonal, transport, and storage proteins.
Protein is the most important component of the human body. Protein is present in all cells making up the enzymes and structural components (tendons, ligaments), contractile proteins (muscle, including the heart), antibodies, hormonal, transport, and storage proteins.
Protein is composed of chains of up to 20 amino acids. Nine amino acids are essential.  This means they must be supplied with the food in sufficient amounts because they cannot be made in the body. The essential amino acids are part of the proteins we eat. Therefore protein is an essential nutrient, without which we cannot survive.
This means they must be supplied with the food in sufficient amounts because they cannot be made in the body. The essential amino acids are part of the proteins we eat. Therefore protein is an essential nutrient, without which we cannot survive.
A complete protein source contains all the essential amino acids; an incomplete protein source lacks one or more of the essential amino acids. More about amino acids here.
In the proteins, the amino acid chains are folded in three dimensions in a very complex way. Thereby each protein achieves its unique structure and function.
Because the body has no protein storage provision it requires a daily supply of proteins to produce new proteins and replace damaged proteins.
Sources

 Meat, fish, and eggs are sources of complete protein. Milk and milk-derived foods are also good sources.
Meat, fish, and eggs are sources of complete protein. Milk and milk-derived foods are also good sources.
Among the best vegetarian sources are soybeans, tofu and other soy products, and legumes (pulses).
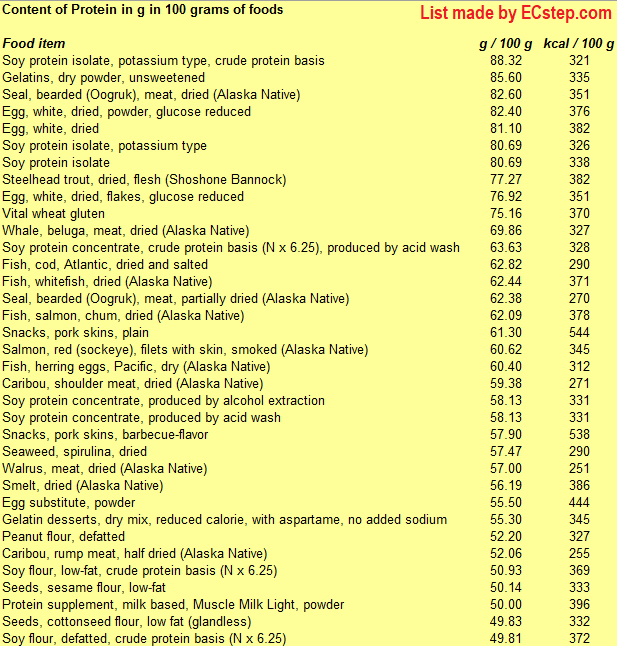
Here is a list of foods having the highest content of protein in g per 100 grams of the food.
Since you may also be interested in foods with a high protein content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
Daily intake
The recommended daily intake of good quality protein is 0.8-1.0 g/kg body weight. If you are physically very active, larger amounts – up to 2.5 g/kg – should be consumed to increase muscle mass, enable body repair, and cover energy needs.
A diet containing adequate amounts of amino acids (especially the essential) is particularly important during early development and maturation, pregnancy, lactation, or injury.
Generally, proteins derived from animal foods (meats, fish, poultry, cheese, eggs, yogurt, and milk) are complete, though gelatin is an exception. Except for quinoa or soybeans, proteins derived from plant foods (legumes, grains, and vegetables) tend to have less of one or more essential amino acids. Corn protein is low in the essential amino acids lysine and tryptophan.
Deficiency
Protein deficiency leads to protein-energy malnutrition with loss of weight. The most severe form is known as Kwashiorkor, which is seen in children who develop edema, irritability, anorexia, skin ulcers, and an enlarged fatty liver. Mental retardation can also be a consequence of severe protein deficiency.
Excess
Provided you drink sufficient water you are unlikely to encounter problems with even very large intakes of protein. However, you should always ensure that your diet contains the other essential nutrients in sufficient amounts.
Protein Structure
Proteins have a very complex structure. They are made up of chains of amino acids in a specific sequence, which is determined by segments of the genetic code in a cell’s DNA. The number of amino acids in the chain can vary from as little as ten to many thousands.
Up to 20 different amino acids can be built into the protein directly from the DNA sequence via messenger RNA. These amino acids are called proteinogenic amino acids.
They include the nine essential amino acids, which must be supplied in the food. They are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
The remaining 11 non-essential amino acids, which can be made in the body from other molecules are alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
Primary structure
The primary structure of proteins refers to the sequence of the amino acids in the amino acids chain or ‘polypeptide’ chain.
![]() The primary structure is held together by chemical bonds made during the synthetic process of the protein taking place in the cells’ cytoplasm at the ribosomes, which is where the amino acid chain is made using the messenger RNA as a template for the formation.
The primary structure is held together by chemical bonds made during the synthetic process of the protein taking place in the cells’ cytoplasm at the ribosomes, which is where the amino acid chain is made using the messenger RNA as a template for the formation.
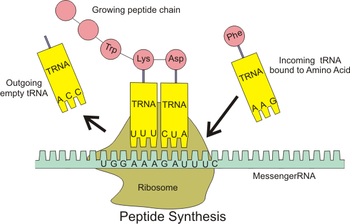 Thus the sequence of bases in messenger RNA (mRNA) – being a copy of the DNA base sequence in the gene for the protein – is translated into the specific amino acid sequence, which determines the special characteristics of the protein in question. In this process transfer RNA (tRNA) is an important intermediary bringing the amino acids to the correct position at the ribosome enabling the formation of the peptide chain (see figure).
Thus the sequence of bases in messenger RNA (mRNA) – being a copy of the DNA base sequence in the gene for the protein – is translated into the specific amino acid sequence, which determines the special characteristics of the protein in question. In this process transfer RNA (tRNA) is an important intermediary bringing the amino acids to the correct position at the ribosome enabling the formation of the peptide chain (see figure).
In our body, there are over 10,000 different proteins. Each protein has its own unique number and sequence of amino acids.
Protein folding is the process by which a protein assumes its functional shape or conformation. It is the physical process by which a polypeptide chain folds into its characteristic and functional three-dimensional structure.
As mentioned above each protein starts as an unfolded polypeptide chain when translated from the sequence of messenger RNA to the linear chain of amino acids.
In this chain, the amino acids interact with each other to produce a well-defined three-dimensional structure, which is the final functional form of the protein. This form is determined by the amino acid sequence (Anfinsen’s dogma).
The final three-dimensional structure of the protein is obtained through the following additional conformations.
Secondary structure
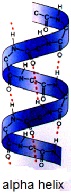
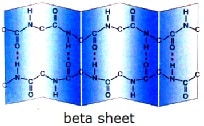 The secondary structure of proteins refers to regular local sub-structures, which are formed in certain areas of the amino acid chain. There are two main types of secondary structure, the alpha helix, and the beta-sheet. They arise due to weak (hydrogen) bonding between certain groups in the amino acid chain. These secondary structures give rise to a regular geometry in certain parts of the protein molecule. Several sequential secondary structures may form a “super secondary unit”.
The secondary structure of proteins refers to regular local sub-structures, which are formed in certain areas of the amino acid chain. There are two main types of secondary structure, the alpha helix, and the beta-sheet. They arise due to weak (hydrogen) bonding between certain groups in the amino acid chain. These secondary structures give rise to a regular geometry in certain parts of the protein molecule. Several sequential secondary structures may form a “super secondary unit”.
Tertiary Structure
 The tertiary structure of proteins refers to the three-dimensional structure of the whole single protein molecule. The alpha-helices and beta-sheets are folded into a compact globule. The folding is driven by the non-specific hydrophobic interactions, but the structure is stable only when the parts of a protein domain are locked into place by specific tertiary interactions, such as salt bridges, hydrogen bonds, and the tight packing of side chains and disulfide bonds.
The tertiary structure of proteins refers to the three-dimensional structure of the whole single protein molecule. The alpha-helices and beta-sheets are folded into a compact globule. The folding is driven by the non-specific hydrophobic interactions, but the structure is stable only when the parts of a protein domain are locked into place by specific tertiary interactions, such as salt bridges, hydrogen bonds, and the tight packing of side chains and disulfide bonds.
Quaternary structure
 The quaternary structure of proteins is the three-dimensional structure of a multi-subunit protein. The final 3D structure is determined by how the subunits fit together. In this context, the quaternary structure is stabilized by the same non-covalent interactions and disulfide bonds as the tertiary structure. Complexes of two or more polypeptides (i.e. multiple subunits) are called multimers. Specifically, it would be called a dimer if it contains two subunits, a trimer if it contains three subunits, and a tetramer if it contains four subunits.
The quaternary structure of proteins is the three-dimensional structure of a multi-subunit protein. The final 3D structure is determined by how the subunits fit together. In this context, the quaternary structure is stabilized by the same non-covalent interactions and disulfide bonds as the tertiary structure. Complexes of two or more polypeptides (i.e. multiple subunits) are called multimers. Specifically, it would be called a dimer if it contains two subunits, a trimer if it contains three subunits, and a tetramer if it contains four subunits.
Proteins consist frequently of several structural subunits.
Structural domains
A structural domain is a part of the protein’s overall structure that is self-stabilizing and often folds independently of the rest of the protein chain. Many domains are not unique to the proteins of one gene or one gene family but instead appear in a variety of proteins. Domains often are named after their biological function in the protein they belong to; for example, the “calcium-binding domain of calmodulin”. Because structural domains are independently stable, they can be “swapped” by genetic engineering between different proteins.
The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded. Failure to fold into the normal structure generally produces inactive proteins, but in some instances, misfolded proteins have a modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins.


