Minerals
4.56/5 (9)
Some elements need to be present in the diet in relatively large amounts. They are usually called macrominerals or “bulk minerals”. They are: Calcium (Ca), Chloride (Cl), Magnesium (Mg), Phosphorus (P), Potassium (K), Sodium (Na), and Sulfur (S). Some are structural, and some play a role as electrolytes.
Many elements are termed trace minerals. They are Chromium (Cr), Cobalt (Co), Copper (Cu), Iodine (I), Iron (Fe), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), Selenium (Se), Zinc (Zn). They are only needed in trace amounts, usually because they play a catalytic role in various enzymatic processes.
Some minerals are present in extremely small amounts (less than 0.0001% by weight) in biological tissues. Nevertheless, they may still have a role to play. They are called ultra trace elements including boron (B), bromine (Br), cadmium (Cd), fluorine (F), lead (Pb), lithium (L), silicon (Si), tin (Sn), and vanadium (V).
You can find more about each of the minerals below.
Calcium
In our body about 1.4% is calcium. Calcium is an essential component of a healthy diet and a mineral necessary for life.
Approximately 99 percent of the body’s calcium is stored in the bones and teeth as calcium phosphate and some calcium sulfate. Calcium can be released from bone by the action of the parathyroid hormone.
The rest of the calcium in the body has other important uses, such as neurotransmitter release and muscle contraction.
Calcium ions are actively pumped out of the cells using adenosine triphosphate (ATP) as an energy source. Calcium ions are among the most widespread second messengers in signaling between cells in neural and muscular tissue.
Vitamin D is needed to absorb calcium.
Sources
 Dairy products, such as milk and cheese, are well-known sources of calcium.
Dairy products, such as milk and cheese, are well-known sources of calcium.
If your are intolerant to lactose (in milk) there are other good sources of calcium including seaweeds such as kelp, wakame and hijiki, nuts and seeds like almonds, hazelnuts, sesame, pistachio, blackstrap molasses, beans, figs, quinoa, okra, rutabaga, broccoli, dandelion leaves, kale, and fortified products such as orange juice and soy milk.
An overlooked source of calcium is eggshells, which can be ground into a powder and mixed into food or a glass of water.
Daily intake
The recommended daily calcium intake for adults is 1000 to 1300 mg. Proper vitamin D status is important for calcium absorption.
The Tolerable Upper Intake Levels (ULs) for calcium are 2.0 to 2.5 grams per day for adults.
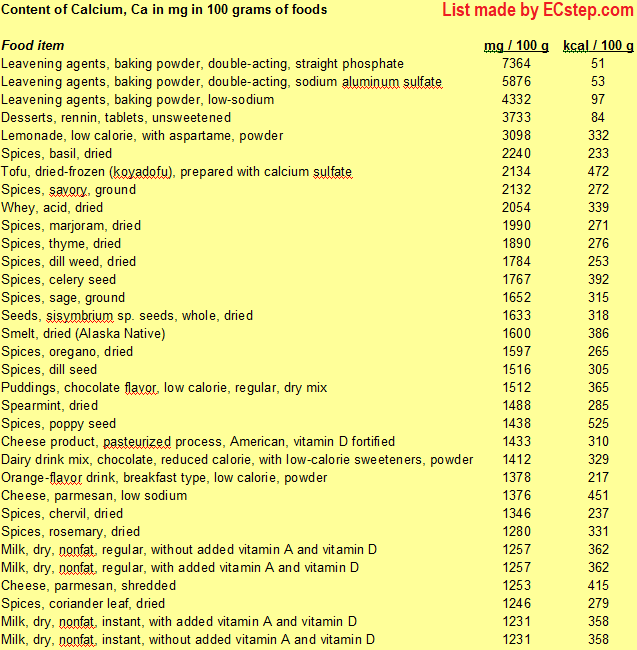
Below is a list of foods having the highest content of calcium in g per 100 grams of the food.
Since you may also be interested in foods with a high calcium content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
Deficiency
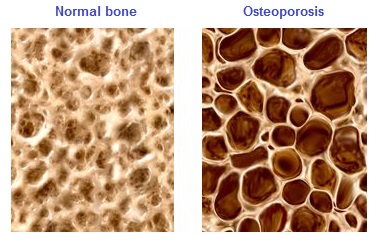 Long-term calcium deficiency (insufficient dietary calcium, insufficient absorption – sometimes due to deficiency of vitamin D) in children and young adults can lead to rickets or osteomalacia (soft bones).
Long-term calcium deficiency (insufficient dietary calcium, insufficient absorption – sometimes due to deficiency of vitamin D) in children and young adults can lead to rickets or osteomalacia (soft bones).
In menopausal women and elderly men calcium deficiency can lead to osteoporosis (porous, brittle bones) with reduced bone mineral density (BMD) and deteriorating microarchitecture leading to an increased risk of fractures.
Calcium deficiency can also lead to poor blood clotting.
Low calcium concentration in the blood (hypocalcemia) may occur. This can lead to tingling or ‘pins and needles’ sensation in and around the mouth and lips, and in the extremities of the hands and feet. Muscular cramps may also occur.
Excess
Compared with other metals, the calcium ion and most calcium compounds have low toxicity.
Over-retention of calcium, which is rare, can cause hypercalcemia (elevated levels of calcium in the blood), impaired kidney function and decreased absorption of other minerals. High calcium intake can cause constipation.
References: 1 , 2 , 3 , 4
–
–
–
Chloride
In our body about 0.15% is chloride. Chlorine can only exist in the body as chloride ions mostly together with sodium ions. Thus chloride is the counterpart of sodium in sodium-chloride or table salt. Both chloride and sodium are essential components of the human body.
Chloride is found mainly in the fluid outside cells, alongside sodium. About 15% of chloride is located inside cells, with the highest amounts in red blood cells. Chloride is also present in very small amounts in bones.
Chloride works with sodium and potassium to maintain the proper balance of body fluids, as well as their pH balance. In addition chloride is an essential component of digestive juices, as it is needed with hydrogen to form hydrochloric acid in the stomach.
Chloride and sodium help maintain proper blood volume and pressure. The concentration of chloride, sodium and potassium in the blood is carefully controlled by the kidneys.
Sources
Most of our chloride intake is from table salt and salt added to many foods during processing or cooking.
Other sources high in chloride are salt substitutes such as potassium chloride, seaweed (such as dulse and kelp), olives, rye, vegetables like celery, lettuce, tomatoes, preserved meats such as bacon, ham, sausages, and processed or canned foods or fast foods that are high in salt.
Daily intake
The North American Dietary Reference Intake recommends a daily intake of between 2300 and 3600 mg/day for 25-year-old males.
Deficiency
Deficiency of chloride can occur with heavy sweating, as large amounts of sodium and chloride can be lost in perspiration. Prolonged diarrhea or vomiting can lead to excessive loss of fluid, chloride, potassium and sodium.
Symptoms of chloride deficiency may also include loss of appetite, muscle weakness, lethargy and dehydration.
Excess
This is normally not a concern since excess chloride is excreted by the kidneys.
However very high intakes of more than 15 grams a day, usually in the form of salt, may lead to symptoms such as acid-base imbalance, fluid retention, and high blood pressure. (These problems are due more to the excess sodium than to the excess chloride.)
References: 1
Chromium
In our body only trace amounts of the essential mineral chromium is present.
Chromium forms part of a compound in the body known as the glucose tolerance factor (GTF), which is involved in regulating the actions of insulin in maintaining blood sugar levels and, possibly, in helping to control appetite.
Chromium also assists in the maintenance of healthy blood levels of cholesterol and other lipids.
Sources
Processed meats, brewer’s yeast, whole grain products, ready-to-eat bran cereals, green beans, broccoli, prunes, mushrooms, spices, and beer are relatively rich in chromium.
Daily intake
The estimated safe and adequate daily dietary intake for chromium is 50 to 200 micrograms. It is estimated that 90% of Americans consume less than the recommended amount of chromium each day.
Chromium supplements have been shown to reduce triglyceride levels by almost 20%, improve glucose tolerance and normalize insulin levels. Supplements of 400 micrograms have helped overweight women lose fat.
Natural forms of supplemental chromium, such as chromium-rich yeast, may be absorbed somewhat more efficiently than inorganic forms of chromium, such as chromium chloride, which is found in some supplements.
Deficiency
Insufficient dietary intake of chromium leads to signs and symptoms that are similar to those observed in diabetes and cardiovascular diseases. Most diets contain less than 60% of the minimum suggested intake of 50 micrograms per day. Chromium is poorly absorbed in the intestines.
Thus a shortage of chromium may lead to glucose intolerance and insulin resistance (particularly in people with diabetes), inadequate metabolism of amino acids, increased risk of arteriosclerosis, anxiety, and fatigue.
Excess
There have been no documented signs of chromium toxicity in any of the nutritional studies at levels up to 1 mg per day.
Because chromium is not easily absorbed (chromium picolinate is the best absorbed supplementation) and since it is lost easily in the urine, toxicity does not seem to be a problem. However, if chromium is taken in large dosages over prolonged periods dermatitis, as well as gastrointestinal ulcers and liver and kidney damage have been seen.
People with liver or kidney disease may be more susceptible to adverse effects from excessive intake of chromium.
Cobalt
In our body only trace amounts of the essential mineral cobalt is present. It is a key constituent of cobalamin or vitamin B12, which is the primary biological reservoir of cobalt. Thus the function of cobalt is interwoven with that of vitamin B12.
Cobalt itself has a few other functions: it helps form the myelin which covers the nerves and it activates a few important enzymes in the metabolism.
Sources
 Dietary sources of cobalt are the same as vitamin B12, such as foods of animal origin. Liver, kidney and heart are rich in cobalt.
Dietary sources of cobalt are the same as vitamin B12, such as foods of animal origin. Liver, kidney and heart are rich in cobalt.
Other good sources are clams, oysters, extra-lean beef, seafood, eggs, milk and yogurt, chicken, and cheese.
Plants and fruits are generally poor in cobalt and vitamin B12.
Daily intake
The recommended daily intake is 2.4 micrograms as vitamin B12.
Deficiency
Vegetarians have a high risk of developing cobalt and vitamin B12 deficiency. For this reason vegetarians are recommended supplements of vitamin B12.
Deficiency can lead to severe anemia, fatigue, circulation problems, gastrointestinal problems, and failure in myelin sheath formation. It may also lead to slow growth rate.
Excess
An excess of cobalt in the form of soluble cobalt salts is very toxic and can lead to cardiac myopathy, goiter and nerve deafness. Cobalt is eliminated from the body almost entirely by the kidneys.
Intake of vitamin B12 in large doses is not toxic.
Reference: 1
–
–
–
Copper
In humans, copper is essential to the proper functioning of many organs and metabolic processes. Copper is incorporated into a variety of proteins and metalloenzymes, which perform essential metabolic functions.
Copper is necessary for the proper growth, development, and maintenance of bone, connective tissue, brain, heart, and many other body organs.
Copper is involved in the formation of red blood cells, the absorption and utilization of iron, the metabolism of cholesterol and glucose, and the synthesis and release of life-sustaining proteins and enzymes, which produce cellular energy and regulate nerve transmission, blood clotting, and oxygen transport.
Copper stimulates the immune system to fight infections, to repair injured tissues, and to promote healing. Copper also helps to neutralize “free-radicals”, which can cause severe oxidative damage to cells.
In addition copper is essential for the normal growth and development of human fetuses, infants, and children.
Sources
The best dietary sources include shellfish, liver, whole grains (wheat and rye), beans, lentils and chocolate.
Nuts, including peanuts and pecans, are rich in copper, as are several fruits including lemons and raisins.
Other food sources that contain copper are cereals, potatoes, peas, red meat, mushrooms, kale, coconuts, papaya and apples.
Daily intake
For adults the recommended daily intake is 0.9 mg. The recommended intake for pregnant women is 1 mg/day and for lactating women it is 1.3 mg/day.
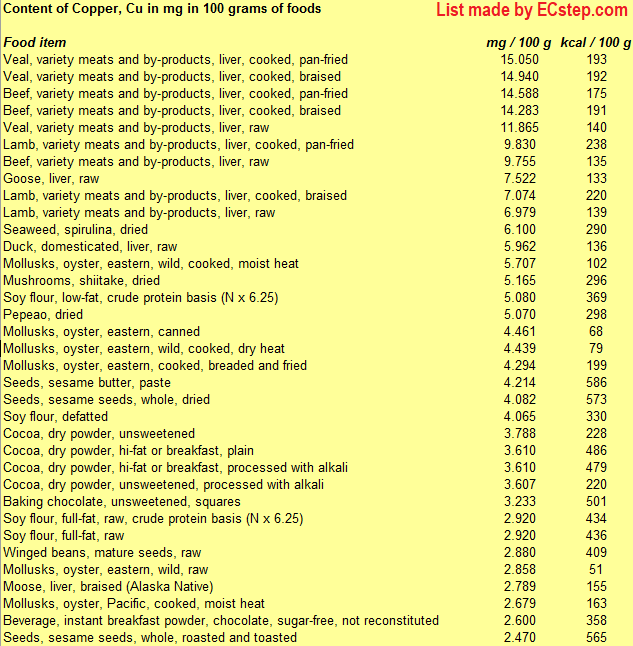
Below is a list of foods having the highest content of copper in g per 100 grams of food.
Since you may also be interested in foods with a high copper content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
Deficiency
 Copper is ubiquitous and the daily requirement is low. Therefore copper deficiency is very rare.
Copper is ubiquitous and the daily requirement is low. Therefore copper deficiency is very rare.
Eating a balanced diet with a range of foods from different food groups is the best way to avoid copper deficiency.
Excess
The maximum tolerable intake is 10 mg/day.
Acute symptoms of copper poisoning by ingestion include vomiting, gastrointestinal bleeding and distress, low blood pressure, jaundice and coma.
Chronic (long-term) effects of copper exposure can damage the liver and kidneys.
Iodine
Iodine is an essential element for life. In our body only trace amounts of iodine is present.
 Iodine is a constituent of the thyroid hormones, thyroxine iodine-containing hormones regulate your metabolic rate(T4) and triiodothyronine (T3). Thyroid hormones regulate the basal metabolic rate in the body.
Iodine is a constituent of the thyroid hormones, thyroxine iodine-containing hormones regulate your metabolic rate(T4) and triiodothyronine (T3). Thyroid hormones regulate the basal metabolic rate in the body.
However, 70% of the body’s iodine is distributed in other tissues, including mammary glands, eyes, gastric mucosa, the cervix, and salivary glands, where it has other important functions.
Sources
Iodized salt – iodide fortified table salt – is the main food source of iodine. Seafood is naturally rich in iodine.
Cod, sea bass, haddock, perch, kelp and plants grown in iodine-rich soil are good sources of iodine. Dairy products also contain iodine.
Daily intake
The recommended daily intake of iodine is 150 micrograms for adults, 220 micrograms for pregnant women and 290 micrograms for lactating women.
These intakes are necessary to ensure a normal basal metabolic rate and optimal function of all processes in which iodine have a role in the body.
Deficiency
Iodine deficiency gives rise to hypothyroidism with extreme fatigue, goitre (swelling in the thyroid gland), mental slowing, depression, weight gain, and low basal body temperatures.
Iodine deficiency is the leading cause of preventable mental retardation, which occurs primarily when babies or small children lack iodine. Iodine deficiency is a serious public health problem in some developing countries.
The addition of iodine to table salt has largely eliminated iodine deficiency in the wealthier nations.
Excess
The tolerable upper intake level for adults is 1100 micrograms/day.
Acute iodine poisoning is rare and occurs only if taken orally in a large amount.
The lethal dose for adults is 2–3 grams of iodine. Symptoms of acute iodine poisoning include burning of the mouth, throat, and stomach, fever, nausea, vomiting, diarrhea, weak pulse and coma.
Iron
Iron is an essential trace element found in nearly all living organisms. In our body about 4 grams of iron is present.
Iron is a key component of important proteins including haemoglobin, myoglobin, and several enzymes, including iron catalase, iron peroxidase, and the cytochrome enzymes, which perform important oxidation and reduction processes.
Almost two thirds of the body’s iron is found in haemoglobin in the red blood cells, which transport oxygen from the lungs to all other tissues in the body. About a quarter of the body’s iron is stored as ferritin or haemosiderin.
The remaining iron is in the muscles’ myoglobin, which stores and distributes oxygen necessary for muscular activity, and in the enzymes mentioned above.
Sources
Iron is pervasive, but particularly rich sources of dietary iron are red meat, lentils, beans, poultry, fish, leaf vegetables, watercress, tofu, chickpeas, black-eyed peas, blackstrap molasses, fortified bread, and fortified breakfast cereals.
Iron in low amounts is found in molasses, teff and farina. Iron in meat is more easily absorbed than iron in vegetables.
Daily intake
The Recommended Dietary Allowance (RDA) for iron varies considerably with age, gender, and source of dietary iron (heme-based iron (in meat) has higher bioavailability).
In adult females before menopause 18 mg/day is recommended. The recommended additional intake is 27 mg/day during pregnancy and 9 mg/day during breast feeding. In adult males and in females after the menopause 8 mg/day is recommended.
Infants may require iron supplements if they are bottle-fed cow’s milk. Blood donors and pregnant women (see above) are at special risk of low iron levels and should supplement their iron intake.
The tolerable upper limit for iron is 40 mg/day for children and 45 mg/day for adults. This limit is set as the largest amount of iron a person can consume long term without risk of negative side effects.
However, if you are deficient in iron, larger amounts (about 100 mg/day) may be taken until the deficiency has been corrected.
Absorption of iron may be enhanced by being taken together with vitamin C (ascorbic acid).
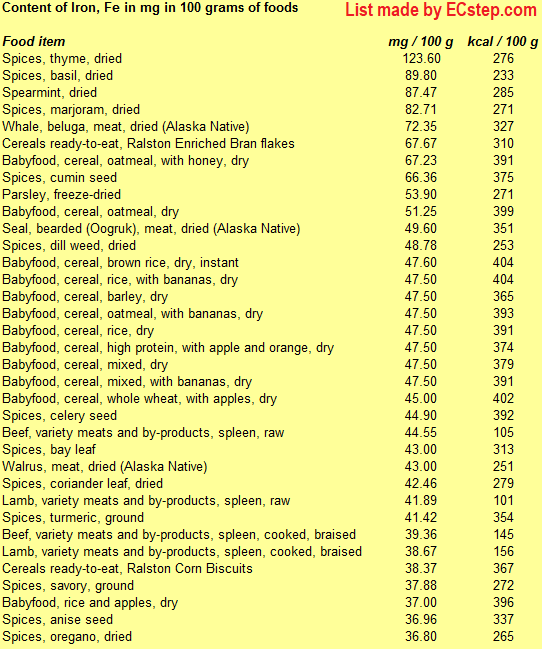
Below is a list of foods having the highest content of iron in g per 100 grams of food.
Since you may also be interested in foods with a high iron content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
Deficiency
 Deficiency can develop from bleeding including excessive menstrual bleeding, bleeding from the gastrointestinal tract (ulcers, hemorrhoids, etc.), and from blood donation.
Deficiency can develop from bleeding including excessive menstrual bleeding, bleeding from the gastrointestinal tract (ulcers, hemorrhoids, etc.), and from blood donation.
It can also be a consequence of inadequate intake (e.g. a vegetarian diet), or of substances in the diet interfering with iron absorption like phytate, especially in bran and seeds.
The main manifestation of iron deficiency is anaemia with symptoms like fatigue, dizziness and pallor. Other symptoms of iron deficiency are hair loss, brittle or grooved nails, irritability and weakness.
In the United States 20% of all women of childbearing age suffer from iron deficiency.
Excess
Because intestinal absorption of iron is regulated by iron stores, iron toxicity is rare. Consuming large quantities of alcohol may increase the absorption of iron.
Once iron is absorbed it is only excreted through blood loss. Excess iron will build up in tissues and organs and may increase the risk for certain cancers and eventually lead to death.
Acute iron intoxication may occur with intakes above 20 mg/kg body weight. The lethal dose is 60 mg/kg body weight. Excess iron damages especially the heart and liver. The medical management of iron toxicity is deferoxamine which binds and expels excess iron from the body.
The main concern with iron toxicity is overdosing in children. It is important to keep iron supplements away from children and tightly closed.
References: 1 , 2 , 3 , 4
–
–
–
Magnesium
Our body contains about 20 grams of the essential nutrient mineral magnesium.
Magnesium occurs typically as the Mg2+ ion and is present in every cell in every organism. Thus ATP (adenosine triphosphate), which is the main source of energy in cells, must be bound to magnesium to be biologically active.
Magnesium plays a role in the stability of polyphosphate compounds including those associated with DNA- and RNA-synthesis.
Over 300 enzymes require the presence of magnesium ions to work. Magnesium is necessary for synthesis of chlorophyll and photosynthesis in plants.
Sources
Green vegetables such as spinach provide magnesium because of the abundance of the green chlorophyll molecules containing magnesium.
Nuts (especially cashews and almonds), seeds, dark chocolate, roasted soybeans, bran, and some whole grains are also good sources of magnesium.
Refined foods are generally poor in magnesium.
Daily intake
The nutritional requirement for adults is 300-400 mg/day.
Eating a wide variety of legumes, nuts, whole grains, and vegetables will meet your dietary need for magnesium.
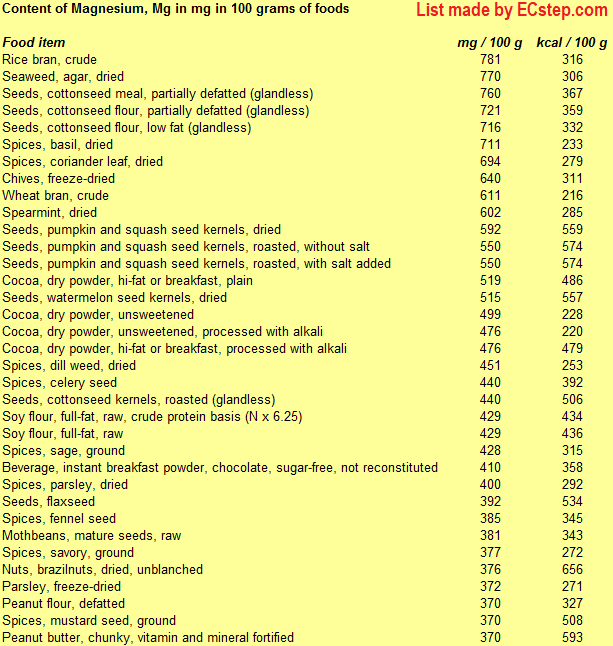
Below is a list of foods having the highest content of magnesium in g per 100 grams of food.
Since you may also be interested in foods with a high magnesium content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
Deficiency
Inadequate magnesium intake frequently causes irregular heartbeats, high blood pressure, insomnia and muscle spasms.
Magnesium deficiency has also been associated with cardiovascular disease, diabetes, anxiety disorders, migraines, osteoporosis, and cerebral infarction.
Acute magnesium deficiency is rare. It is more common as a side effect of chronic alcohol or diuretic use than from low intake alone.
Excess
Dietary magnesium does not pose a health risk.
However, high doses of magnesium in supplements can lead to adverse effects such as diarrhea and abdominal cramps.
The risk of magnesium toxicity increases with kidney failure, when the kidneys lose the ability to remove excess magnesium.
Oral magnesium poisoning in adults with normal renal function is very rare.
Too much magnesium may make it difficult for the body to absorb calcium.
References: 1 , 2 , 3 , 4
–
–
–
Manganese
Manganese is an essential trace nutrient in all forms of life. The human body contains about 12 mg of manganese.
In the human body, manganese functions as an enzyme activator and as a component of metalloenzymes (enzymes that contain a metal ion in their structure).
Manganese helps in the utilization of several key nutrients including biotin, thiamin, ascorbic acid, and choline.
It is a catalyst in the synthesis of fatty acids and cholesterol, and in the metabolism of protein and carbohydrate.
Manganese helps maintain reproductive health, bone formation, thyroid hormone production, health of nervous system, and mitochondrial function.
Sources
Foods rich in manganese include whole grains (including buckwheat, bulgur wheat, oats), nuts, and seeds, wheat germ, pineapples and legumes.
Please note that refined grains only provide half the amount of manganese found in whole grains.
Daily intake
A manganese intake of 2 mg/day seems sufficient to prevent deficiency in most individuals.
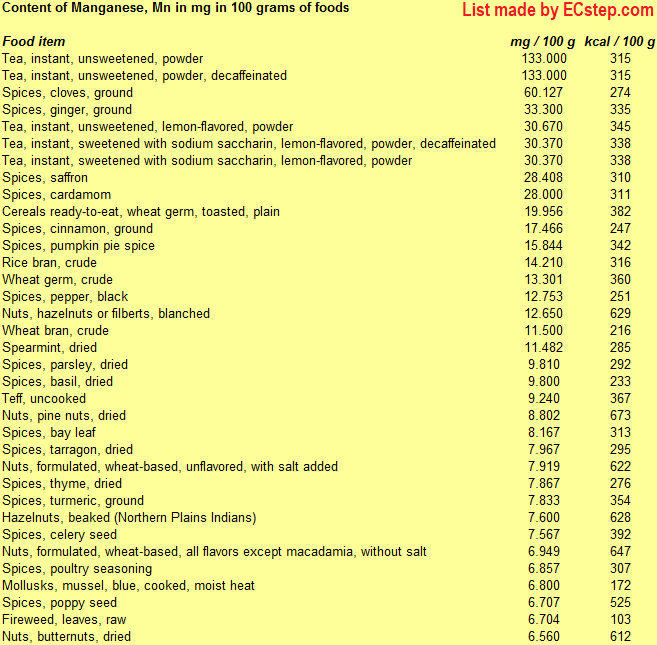
Below is a list of foods having the highest content of manganese in g per 100 grams of the food.
Since you may also be interested in foods with a high manganese content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
Deficiency
Low levels of manganese in the body can contribute to infertility, bone malformation, weakness, and seizures.
Many Americans do not get the recommended dietary intake of manganese in their diet, which tends to contain more refined grains than whole grains.
Excess
Too much manganese in the diet can lead to high levels of manganese in the body tissues, especially in the brain.
This may lead to neurological problems. In the young a poor development of the nervous system with impaired cognitive performance, and hyperactive and oppositional behaviour can occur.
The elderly may develop neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis more frequently.
Molybdenum
In our body, the essential nutrient molybdenum is only present in trace amounts.
The molybdenum cofactor molecule is the structure in which almost all molybdenum-containing enzymes (molybdoenzymes) work.
Molybdenum has a role in the metabolism of sulfur-containing amino acids (methionine and cysteine), in the breakdown of nucleotides (precursors for DNA and RNA) to uric acid, and in the metabolism of drugs and toxins.
Sources
Good sources of molybdenum are pork, lamb and beef liver, eggs, beans, lentils, peas, sunflower seeds, cucumbers, and grain products.
Daily intake
The average daily intake of molybdenum varies between 120 and 240 micrograms, depending on the molybdenum content of the food.
The recommended daily intake for adults is only 45 micrograms.
The tolerable upper level of intake for adults is 2000 micrograms per day.
Deficiency
Dietary molybdenum deficiency has never been observed in healthy people.
Excess
Acute toxicity has not been seen in humans.
Animal studies have indicated that chronic ingestion of more than 10000 micrograms per day of molybdenum can cause diarrhea, growth retardation, infertility, low birth weight and gout. It may also affect the lungs, kidneys, and liver.
Nickel
An average adult person contains only about 10 milligrams of nickel, which is an essential nutrient.
Nickel is built into certain important enzymes in the body. Nickel seems also involved in the production and action of some hormones.
Nickel helps iron absorption and improves bone strength.
It assists in red blood cell production, skin maintenance, and optimal growth. It also has functions in the adrenaline and glucose metabolism.
Sources
Sources of nickel are oats, peas, beans, nuts, and chocolate.
Daily intake
The recommended daily intake is 100 milligrams.
Supplementation is seldom necessary.
Deficiency
Nickel deficiency is rare.
Deficiency may be associated with low blood glucose levels, abnormal bone growth, altered metabolism of calcium, and poor absorption of iron.
Excess
Toxicity by excessive consumption is rare and would require 1000 times the amount normally consumed in food.
However, an excessive exposure from the environment, leading to toxicity, is common, e.g. from tobacco, dental implants, stainless steel kitchen utensils and inexpensive jewellery.
Overexposure to the metal is most prevalent in people involved in nickel mining or processing.
Toxicity may result in changes in glucose tolerance, blood pressure, response to stress, growth rate, bone development, and resistance to infection.
Nickel (Jewelry) Allergy
If earrings make your earlobes itch or your necklace leaves a rash around your neck, you may have a nickel allergy. It’s one of the most common skin allergies, in part because nickel is used in everything from jewelry to cell phones, coins, zippers, and eyeglass frames.
The most important thing to do is to avoid skin contact with objects having nickel in them.
Phosphorus
An average adult human contains about 700 grams of the essential nutrient phosphorus in the form of phosphate, which is required for all known forms of life.
 Biological molecules such as DNA and RNA include phosphate as a part of the structural framework of these molecules.
Biological molecules such as DNA and RNA include phosphate as a part of the structural framework of these molecules.
 Living cells use phosphate to produce cellular energy in the form of adenosine triphosphate (ATP), which can be utilized to drive energy-consuming processes in the body.
Living cells use phosphate to produce cellular energy in the form of adenosine triphosphate (ATP), which can be utilized to drive energy-consuming processes in the body.
Phospholipids are important structural components of all cell membranes.
Calcium phosphate salts assist in stiffening bones and teeth in the form of apatite. About 85% of the body’s phosphorus is in the bones and teeth.
Sources
Good sources of phosphorus are foods rich in protein, for example, milk, eggs, dairy products, meat, poultry, fish, nuts, and legumes.
Daily intake
The recommended daily intake of dietary phosphorus for adults is 700 mg.
Normally you do not need to take phosphorus supplements. If your diet contains sufficient amounts of protein and calcium, then the amount of phosphorus should also be sufficient.
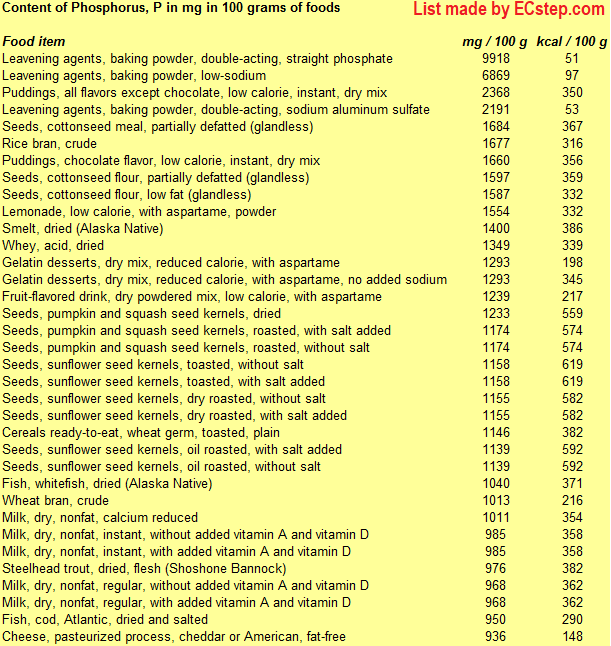
Below is a list of foods having the highest content of phosphorus in g per 100 grams of the food.
Since you may also be interested in foods with a high phosphorus content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
Deficiency
Some health conditions such as diabetes, starvation, and alcoholism can cause levels of phosphorus in the body to fall.
The same is true of conditions that make it hard for people to absorb nutrients, such as Crohn’s disease and celiac disease. All are characterized by hypophosphatemia (low concentration of phosphate in the blood).
 The symptoms of phosphorus deficiency include loss of appetite, anxiety, bone pain, fragile bones, stiff joints, fatigue, irregular breathing, irritability, numbness, weakness, and weight change.
The symptoms of phosphorus deficiency include loss of appetite, anxiety, bone pain, fragile bones, stiff joints, fatigue, irregular breathing, irritability, numbness, weakness, and weight change.
In children, decreased growth and poor bone and tooth development may occur.
Excess
Too much phosphorus in the body is generally caused by kidney disease or by consuming too much phosphorus and too little calcium in the diet.
High intakes of phosphorus may be associated with an increased risk of cardiovascular disease.
As the amount of phosphorus you eat rises, so does the need for calcium. The delicate balance between calcium and phosphorus is necessary for proper bone density and the prevention of osteoporosis.
Intake of too much phosphate can lead to diarrhea and calcification (hardening) of organs and tissues. It can also interfere with the body’s ability to utilize iron, calcium, magnesium, and zinc.
Potassium
In our body about 0.25% is potassium. A 70 kg adult contains a total of about 175 g of potassium.
 Potassium is necessary for the function of all living cells. It is the major cation (positive ion) inside cells at a concentration of about 150 mmol/L.
Potassium is necessary for the function of all living cells. It is the major cation (positive ion) inside cells at a concentration of about 150 mmol/L.
Potassium is actively pumped into the cells while sodium is actively pumped out of the cells. This process is mediated by the so-called Na+/K+-ATPase pump.
The resultant concentration differences between the inside and outside of the cell membrane cause what is known as the membrane potential making possible the propagation of impulses in the brain, nerves, and muscles including the heart.
Sources
Eating a variety of foods that contain potassium is the best way to get an adequate amount.
Foods with high sources of potassium include kiwifruit, orange juice, potatoes, bananas, coconut, avocados, apricots, parsnips, and turnips.
Many many other fruits, vegetables, legumes, and meats also contain potassium.
Daily intake
Healthy individuals who eat a balanced diet rarely need supplements.
The recommended daily intake is about 4-5 grams in adults.
However, the Western diet, in general, is rather poor in potassium (about 2 grams per day).
Increasing potassium intake to 4-5 grams per day could ameliorate symptoms associated with potassium deficiency.
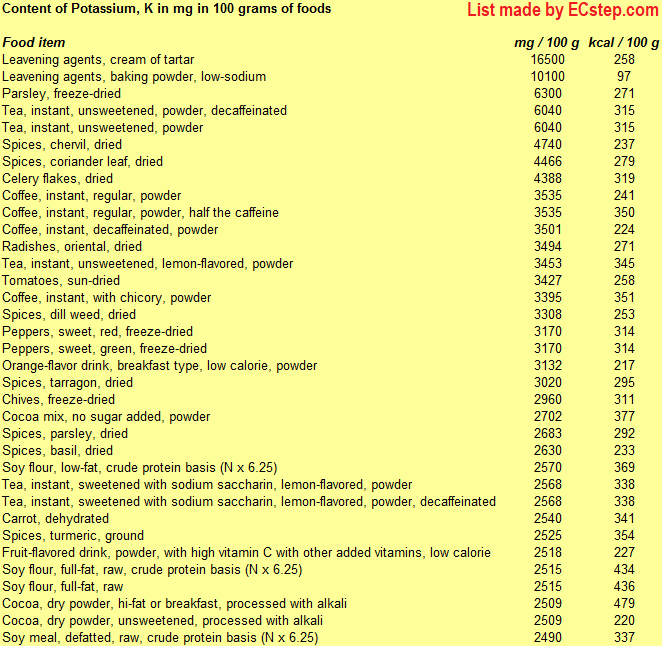
Below is a list of foods having the highest content of potassium in g per 100 grams of the food.
Since you may also be interested in foods with a high potassium content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
Deficiency
A severe shortage of potassium may cause the condition known as hypokalemia (decreased concentration of potassium in the blood). Hypokalemia typically results from loss of potassium through diarrhea, diuresis, or vomiting.
Symptoms are related to alterations in the cells’ membrane potential and metabolism and include muscle weakness and cramps, abnormalities in the electrocardiogram (ECG), intestinal paralysis, decreased reflex response, and (in severe cases) abnormal heart rhythm (arrhythmia).
Excess
Hyperkalemia (elevated concentration of potassium in the blood) is the most serious consequence of a too high potassium intake. Hyperkalemia occurs when potassium builds up in the blood faster than the kidneys can remove it. It is most common in individuals with reduced renal function.
Symptoms of hyperkalemia may include tingling of the hands and feet, muscular weakness, and temporary paralysis.
The most serious effect of hyperkalemia is the development of an abnormal heart rhythm (arrhythmia), which can lead to cardiac arrest.
Although hyperkalemia is rare in healthy individuals, oral doses greater than 18 grams taken at one time in individuals not accustomed to high intakes can lead to hyperkalemia.
Selenium
Selenium is a trace mineral that is essential to good health. Only small amounts are required. The human body’s content of selenium is believed to be in the 13–20 milligram range. Small amounts are necessary for certain cellular functions.
Selenium is incorporated into proteins to make selenoproteins, some of which are important antioxidant enzymes that help reduce certain oxidized molecules and thereby help prevent cellular damage from free radicals.
Some selenoproteins help regulate thyroid function and play a role in the immune system.
Sources
 Dietary selenium comes from nuts, cereals, meat, mushrooms, fish, and eggs.
Dietary selenium comes from nuts, cereals, meat, mushrooms, fish, and eggs.
Brazil nuts are the richest ordinary dietary source (though this is soil-dependent). High levels are also found in kidneys, tuna, crab, and lobster.
Daily intake
The recommended daily intake is 55 micrograms per day in adults.
Below is a list of foods having the highest content of selenium in g per 100 grams of the food.
Since you may also be interested in foods with a high selenium content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
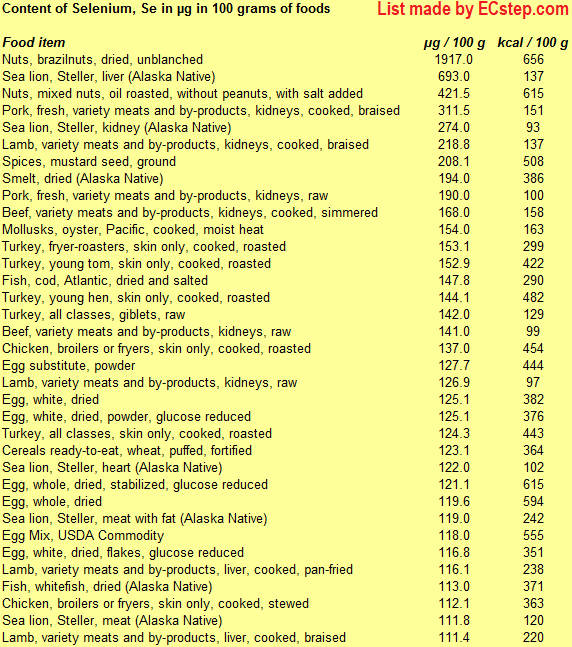
Deficiency
Selenium deficiency is rare in healthy well-nourished individuals. Only few cases have been reported.
However, people dependent on food grown from selenium-deficient soil are at risk. There is evidence that selenium deficiency may contribute to the development of a form of heart disease, hypothyroidism, and weakening of the immune system.
Usually, selenium deficiency does not cause illness by itself. Rather, it can aggravate conditions caused by other nutritional, biochemical, or infectious stresses.
Excess
Exceeding the Tolerable Upper Intake Level of 400 micrograms per day can lead to selenosis.
Symptoms of selenosis include a garlic odor on the breath, gastrointestinal disorders, hair loss, sloughing of nails, fatigue, irritability, and neurological damage.
Extreme cases of selenosis can result in cirrhosis of the liver, pulmonary edema, and death.
Sodium
Sodium is an essential nutrient having important roles in the regulation of blood and body fluids, the transmission of nerve impulses, heart activity, and certain metabolic functions.
Sodium participates in the regulation of blood volume, blood pressure, osmotic equilibrium, and pH. Sodium is also important in neuron function and osmoregulation between cells and the extracellular fluid.
Sodium is actively pumped out of cells. (Potassium is actively pumped into cells.) So the sodium concentration is low inside cells and high outside cells in the extracellular fluid, where sodium is the most prominent positive ion.
The concentration of sodium in the extracellular fluid is about 140 mmol/liter. (This is probably a concentration close to that in the primordial ocean where the first unicellular organisms developed.)
In an average human weighing 70 kg, the extracellular fluid (including the fluid of blood) amounts to about 15 liters, which carry around 50 grams of sodium or 90% of the body’s total sodium content. Here sodium exists as sodium chloride.
Sources
 Sodium chloride is the principal source of sodium in the diet. Sodium chloride is used as seasoning and preservative, like for pickling and jerky.
Sodium chloride is the principal source of sodium in the diet. Sodium chloride is used as seasoning and preservative, like for pickling and jerky.
Most of the ingested sodium comes from processed foods, including condiments and seasonings such as Worcestershire sauce, soy sauce, and bouillon cubes.
Fast foods are generally high in sodium.
Daily intake
 The recommended intake of sodium as sodium chloride is 2.3 grams per day.
The recommended intake of sodium as sodium chloride is 2.3 grams per day.
The Western diet usually contains too much (about 3-4 grams per day).
Below is a list of foods having the highest content of sodium in g per 100 grams of the food.
Since you may also be interested in foods with a high sodium content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
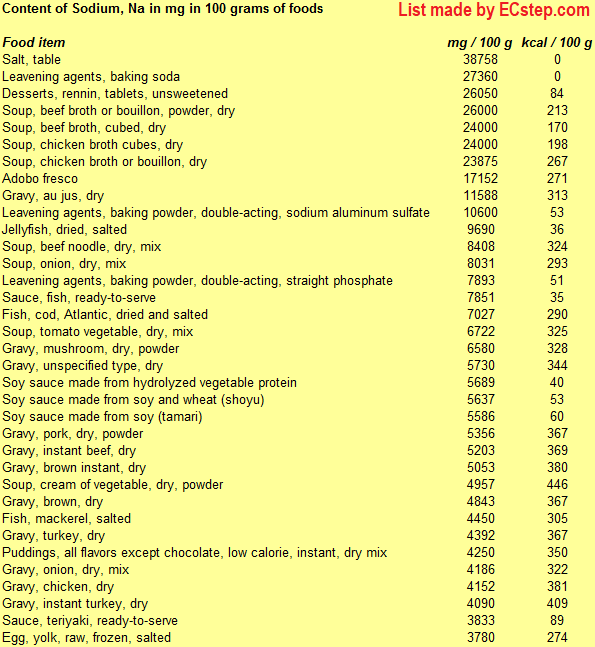
Deficiency
The minimum physiological requirement for sodium is 0.5 grams per day.
Since plants contain little sodium a completely plant-based diet will be very low in sodium.
Deficiency will lead to low concentration in the blood (hyponatremia), which can give rise to nausea and vomiting, headache, confusion, lethargy, fatigue, appetite loss, restlessness, and irritability.
A more serious deficiency can lead to muscle weakness, spasms, cramps, seizures, and decreased consciousness or coma.
Hyponatremia may occur in individuals competing in very long endurance exercise events, such as marathons, ultramarathons, and Ironman triathlons.
The hyponatremia seems to be related to relative fluid overload suggesting a decreased fluid excretion and/or an overestimation of the fluid needs of these athletes.
Excess
A diet containing more than 3-4 grams per day of sodium chloride promotes the development of high blood pressure (hypertension). Hypertension causes 7-8 million premature deaths worldwide.
A high concentration of sodium in the blood (hypernatremia) can result from massive salt ingestion (e.g. by drinking seawater). Most frequently hypernatremia is caused by a relative deficit of water or dehydration due to insufficient intake of water.
Normally even a small rise in the serum sodium concentration will result in a sensation of thirst leading to increased water intake, which will normalize the sodium concentration in the blood.
Therefore, hypernatremia most often occurs in people with an insufficient sensation of thirst or insufficient ability or motivation to drink the necessary amount of water (infants, elderly people, people with impaired mental status).
Sulfur
In our body, there are about 140 grams of sulfur, which is an essential component of all living cells. Since sulfur is built into two amino acids (methionine and cysteine), it is present in many polypeptides, proteins, and enzymes.
 Disulfide bonds (S-S bonds) formed between peptide chains are very important in protein assembly and structure. These bonds between peptide chains confer extra toughness and rigidity.
Disulfide bonds (S-S bonds) formed between peptide chains are very important in protein assembly and structure. These bonds between peptide chains confer extra toughness and rigidity.
For example, the high strength of hair is in part due to its high content of S-S bonds. The same applies to the birds’ feathers. Therefore eggs are high in sulfur being necessary for feather formation.
Sulfur is also a major component of cartilage and skin having a protective, supporting, and structural function. Sulfur is an important part of the substances that support tissues in the body.
Sulfur is a component of one of the main antioxidant protectors called glutathione. Sulfur is also a component of various enzymes that help the body eliminate and deactivate many kinds of toxins.
Sources
 Sulfur is widely available in foods and you can easily get adequate amounts from your diet.
Sulfur is widely available in foods and you can easily get adequate amounts from your diet.
Sulfur occurs primarily in protein-rich foods, including eggs, milk products, meat, and fish.
It is also found in some legumes and in some of the more odiferous vegetables, such as onions, garlic, cabbage, brussel sprouts, and turnips.
Daily intake
There is no recommended sulfur requirement. You will easily get adequate amounts from your diet
Deficiency
A deficiency of sulfur does not seem to occur.
Excess
It is almost impossible to get too much sulfur in the diet.
Reference: 1
–
–
–
Zinc
Zinc is an essential trace mineral that is found in almost every cell. There are 2–4 grams of zinc in the human body. It is a part of more than 100 enzymes.
Zinc is important for a healthy immune system, healthy skin, wound healing, taste, and smell, DNA synthesis and function, and normal growth and development during pregnancy, childhood, and adolescence.
In the brain, zinc plays a key role in the normal functioning of the brain and central nervous system. The body has no zinc storage.
Sources
 Oysters, lobster, and red meats, especially beef, lamb, and liver have some of the highest concentrations of zinc.
Oysters, lobster, and red meats, especially beef, lamb, and liver have some of the highest concentrations of zinc.
Zinc absorption is greater in a diet high in animal protein than in a diet rich in plant proteins.
Phytates, which are found in whole-grain bread, cereals, legumes, and other products, can inhibit zinc absorption.
When there is adequate zinc in the soil, the food plants that contain the most zinc are wheat (germ and bran) and various seeds (sesame, poppy, alfalfa, celery, mustard).
Zinc is also found in beans, nuts, almonds, whole grains, pumpkin seeds, sunflower seeds, and blackcurrant.
Daily intake
The recommended intake is 8 mg/day for women and 11 mg/day for men.
If you need zinc supplementation, zinc glycinate may be preferable since it is particularly well absorbed.
Supplementation should probably not exceed 20 mg/day in healthy people. U.S. National Research Council has set a Tolerable Upper Intake of 40 mg/day.
Below is a list of foods having the highest content of zinc in g per 100 grams of the food.
Since you may also be interested in foods with a high zinc content AND few calories, the list also includes the number of calories.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
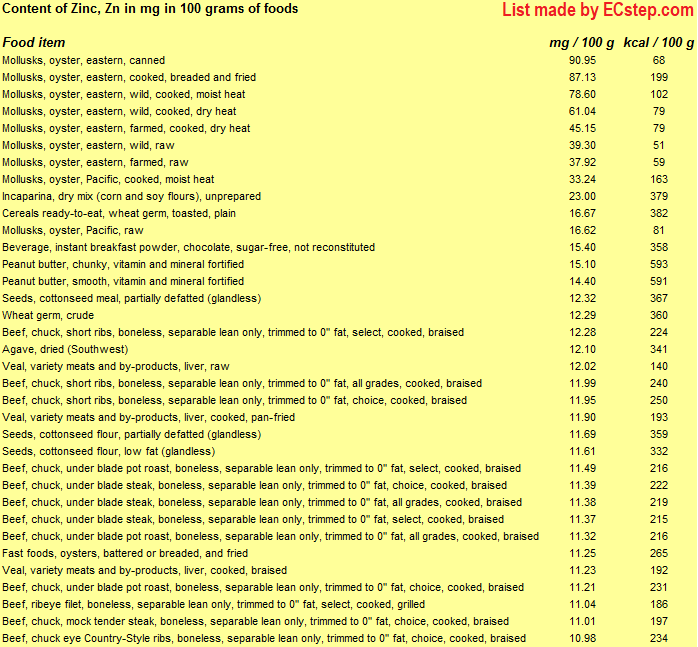
Deficiency
Zinc deficiency can lead to depressed growth, diarrhea, impotence and delayed sexual maturation, alopecia, eye and skin lesions, delayed wound healing, impaired appetite, altered cognition, and impaired host defense.
Excess
Acute adverse effects of high zinc intake include nausea, vomiting, loss of appetite, abdominal cramps, diarrhea, and headaches. Chronic high intake of zinc may adversely affect urinary physiology.
Ultratrace Elements
The ultra-trace elements are present in extremely small amounts (less than 0.0001% by weight) in biological tissues. They include boron (B), bromine (Br), cadmium (Cd), fluorine (F), lead (Pb), lithium (L), silicon (Si), tin (Sn), and vanadium (V).
Boron
No deficiency syndrome has been described in humans. Small amounts of boron occur widely in the diet. Boron occurs in all foods produced from plants. Since 1989 its nutritional value has been argued. It is thought that boron may play some minor biochemical roles in animals, including humans. Supplemental boron may reduce the excretion of calcium, estrogen, and vitamin D and may thereby suppress osteoporosis ( 1).
Bromine
Bromine has no known essential role in human or mammalian health, but inorganic bromine and organobromine compounds do occur naturally. Some organobromine compounds may be of use to higher organisms in dealing with certain parasites and bacteria. Marine organisms are the main source of organobromine compounds.
Elemental bromine is toxic and causes burns. Bromine gas is pale brown, smells like bleach, and is very irritating to mucous membranes. Upon exposure, one should move to fresh air immediately. If symptoms of bromine poisoning arise, medical attention is needed ( 2).
Cadmium
Cadmium has no known useful role in higher organisms, but a cadmium-dependent carbonic anhydrase has been found in some marine diatoms (a form of plankton).
For humans, cadmium is mainly an environmental hazard, as it can irritate the lungs and accumulate in the kidneys. Environmental cadmium is mainly the result of fossil fuel combustion. It is also a byproduct of various industrial processes.
Bread, root crops, and vegetables also contribute to cadmium exposure in modern populations.
However, tobacco smoking is the most important single source of cadmium exposure in the general population. On average, smokers have 4–5 times higher blood cadmium concentrations and 2–3 times higher kidney cadmium concentrations than non-smokers.
Cadmium exposure is a risk factor associated with early atherosclerosis and hypertension, which can both lead to cardiovascular disease.
Due to the adverse effects on the environment and human health, the supply and use of cadmium are restricted in Europe under the REACH Regulation ( 3).
Fluorine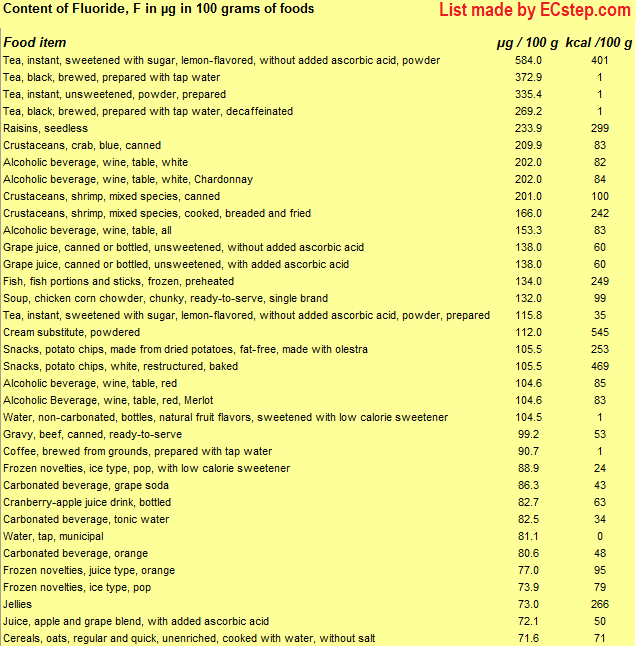
Even if fluoride is not essential for humans, small amounts of fluoride may be beneficial for bone strength. Fluoride ions in contact with teeth are thought to limit cavities by turning the forming of hydroxyapatite of teeth into less soluble fluorapatite. This process works only by direct contact (topical treatment). Fluoride ions that are swallowed do not benefit the teeth.
The addition of fluoride to public water supplies (water fluoridation) is used by about two-thirds of the U.S. Population. Fluorides are commonly used in toothpaste. Fluoride or fluorine deficiency may cause increased dental caries and possibly osteoporosis ( 4).
Here is a list of foods having the highest content of fluoride in g per 100 grams of the food.
The list is made using ECstep’s Personal Nutrition Data Program. Commercial brand products are not included in this list.
Lead
Lead has no known biological function in humans. On the contrary, lead is highly poisonous, affecting many functions in the body, especially in the central nervous system, the cardiovascular system, kidneys, immune system, and bone marrow. It can give rise to nephropathy, colic-like abdominal pains, and weakness in fingers, wrists, or ankles. Lead exposure also causes small increases in blood pressure, particularly in middle-aged and older people, and can cause anemia. Exposure to high lead levels can severely damage the brain and kidneys and ultimately cause death. In pregnant women, high levels of exposure to lead may cause miscarriage. In males, fertility is reduced.
Most exposure occurs through ingestion or inhalation. Lead poisoning typically results from ingestion of food or water contaminated with lead, but it may also occur after accidental ingestion of contaminated soil, dust, or lead-based paint.
By the mid-1980s, a significant shift in lead end-use patterns had taken place. Much of this shift was a result of the U.S. lead consumers’ compliance with environmental regulations that significantly reduced or eliminated the use of lead in non-battery products, including gasoline, paints, solders, and water systems. Lead use is being further curtailed by the European Union’s RoHS directive. Lead may still be found in harmful quantities in stoneware, vinyl (such as that used for tubing and the insulation of electrical cords), and Chinese brass. Lead salts used in pottery glazes have on occasion caused poisoning, when acidic drinks, such as fruit juices, have leached lead ions out of the glaze.
Acute lead poisoning is treated using disodium calcium edetate: the calcium chelate of the disodium salt of ethylene-diamine-tetraacetic acid (EDTA). This chelating agent has a greater affinity for lead than for calcium and so the lead chelate is formed by an exchange. This is then excreted in the urine leaving behind harmless calcium ( 5).
Lithium
Lithium has no known role in normal biology. Nevertheless, lithium has been demonstrated to have a mood-stabilizing effect. So a number of salts of lithium are being used in the treatment of the bipolar disorder, where they have a role in the treatment of depression and particularly of mania, both acutely and in the long term. Lithium is probably more effective in preventing mania than depression. It reduces the risk of suicide in bipolar patients.
Upon ingestion, lithium becomes widely distributed in the central nervous system and interacts with a number of neurotransmitters and receptors, decreasing norepinephrine release and increasing serotonin synthesis.
Lithium toxicity may occur in persons taking excessive amounts. The manifestations include nausea, vomiting, diarrhea, confusion, polyuria, seizures, and coma. Other toxic effects of lithium include tremors, muscle twitching, convulsions, and renal failure. Lithium is also believed to permanently affect renal function, although this does not appear to be common ( 6).
Silicon
Although silicon is readily available in the form of silicates, very few organisms have a use for it. In plants, silicon has been shown to improve cell wall strength and structural integrity. Its exact function in the biology of animals is still under discussion.
Silicon is known to be needed for the synthesis of elastin and collagen. It promotes strength in the hair, skin, and nails and helps maintain the elasticity of the skin. Silicon is present in bone, blood vessels, cartilage, and tendons, helping to make them strong. Silicon is important to bone formation, as it is found in active areas of calcification.
If you want to get extra silicon, eat more whole grains and fresh vegetables or use herbs, such as horsetail, alfalfa, or comfrey ( 7).
Tin
Tin has no known natural biological role in living organisms. It is not easily absorbed by animals and humans. The low toxicity is relevant to the widespread use of tin in dinnerware and canned food. Nausea, vomiting, and diarrhea have been reported after ingesting canned food containing 200 mg/kg of tin. A study showed that 99.5% of the controlled food cans contain tin in an amount below that level.
Organotin compounds with various organic groups can be very toxic. They are used as powerful agents to destroy bacteria, fungi, mites, and ticks ( 8).
Vanadium
Vanadium plays a very limited role in biology and is more important in ocean environments than on land. Eating fish and using vegetable oils in the diet will usually supply sufficient vanadium.
Rats and chickens are known to require vanadium in very small amounts and deficiencies result in reduced growth and impaired reproduction. Vanadium may increase insulin sensitivity and thereby improve glucose control in people with type 2 diabetes.
All vanadium compounds should be considered toxic. However, vanadium compounds are poorly absorbed from the gastrointestinal tract reducing the risk of toxicity. Inhalation exposures to vanadium and vanadium compounds result primarily in adverse effects on the respiratory system.


